INTRODUCTION
Enterovirus 71 (EV71), like poliovirus, is one of the 69 enterovirus serotypes belonging to the Picoranviridae family. EV71 infection manifests most frequently as hand, foot and mouth disease (HFMD) and children under 5 years of age are particularly susceptible to the most severe forms of EV71-associated neurological disease, including aseptic meningitis, brainstem encephalitis and acute flaccid paralysis indistinguishable from poliomyelitis[1]. The neurovirulence of EV71 first came to people's attention in 1975 in Bulgaria when 44 people died of a polio-like disease[2]. EV71 was further implicated as the causative agent responsible for the epidemics of central nervous system disease occurring in New York, Europe, Australia and Asia[3-6]. Recent HFMD outbreaks also affected thousands of children in Taiwan[7], Japan[8] and Singapore[9], causing the fatality of more than 80 children. Thus EV71 appears to emerge as an increasingly important neurotropic enterovirus in the upcoming era of poliomyelitis eradication. However, no effective antiviral drugs or vaccines are available yet.
EV71 possesses a positive single-stranded RNA genome that consists of a single open reading frame (ORF). The ORF expresses a large polyprotein that can be co- and post-translationally cleaved into P1, P2 and P3 regions[10]. P1 region encodes the four structural proteins VP1, VP2, VP3 and VP4, while P2 and P3 regions encode other nonstructural proteins (e.g., 2A and 3CD) responsible for virus replication and virulence[1]. Based on a model derived from poliovirus, protease 2A autocatalytically cleaves P1 at its N-terminus and liberates P1 from the nascent polyprotein[11], while protease 3CD cleaves P1 precursor into VP1, VP3 and VP0 in trans[12]. These three structural proteins spontaneously assemble into icosahedral procapsid in an ordered manner and proceed through a series of intermediates, followed by the encapsidation of the RNA genome into the provirion[13]. The final encapsidation step involves the cleavage of VP0 into VP2 and VP4, therefore the final mature virion consists of 60 copies each of VP1 and VP3, 58-59 copies of VP2 and VP4 and 2-1 copies of VP0[13].
Besides the mature virions, naturally occurring virus-like particles (VLPs) of poliovirus have been discovered[14]. VLPs are empty particles consisting of viral structural proteins but devoid of viral nucleic acids, hence they are non-infectious. VLPs can generally induce broad and strong immune responses thanks to the preservation of many essential epitopes[15-17], therefore VLPs have captured increasing attention as potential vaccine candidates[17-19]. To date, numerous VLPs of clinically important viruses, including human immunodeficiency virus[20], SARS coronavirus[21] and hepatitis delta virus[22], have been expressed. In addition, recombinant poliovirus VLPs have been produced using the baculovirus/insect cell or vaccinia virus expression system. These particles were produced via the (1) expression of the complete ORF; (2) co-expression of individual VP0, VP1 and VP3 proteins; or (3) co-expression of P1 and 3CD proteins[23-25]. Since EV71 and poliovirus share identical genome organization and similar protein functions, we hypothesized that EV71 assembly occurs in a way similar to poliovirus and have previously demonstrated the formation of EV71 VLP by co-infecting insect cells with two recombinant baculoviruses, Bac-P1 expressing P1 and Bac-3CD expressing 3CD[26]. However, the factors influencing the VLP yield were not investigated, and the VLPs were not purified or characterized. In this study, we extended our previous work by further constructing a new recombinant baculovirus simultaneously expressing P1 and 3CD. The new virus was used for single infection of insect cells, co-expression of P1 and 3CD and synthesis of VLP. The yields of VLP produced via the single infection and co-infection strategies were compared. Finally, the VLPs were purified and characterized.
MATERIALS AND METHODS
Cell culture and media
Spodoptera frugiperda (Sf-9) insect cells were cultured in spinner flasks (Bellco) at 27 °C using TNM-FH medium supplemented with 100 mL/L fetal bovine serum (FBS, Gibco BRL) as described elsewhere[27].
Generation of recombinant baculoviruses
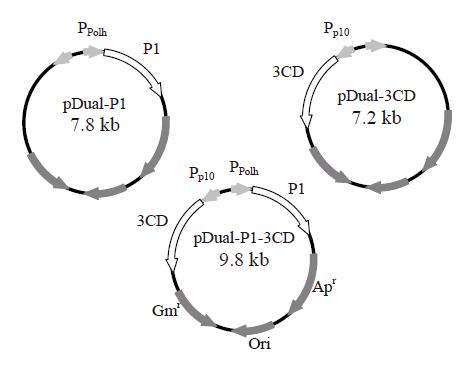
The gene fragments coding for P1 and 3CD were amplified by polymerase chain reaction from the full-length cDNA clone of EV71 neu strain[28] and subcloned separately into multiple cloning site (MCS) I and II of pFastBacTM DUAL plasmids (Invitrogen) as described previously[26]. The resultant plasmids were designated pDual-P1 and pDual-3CD, respectively. Likewise, the P1 gene fragment was cloned into MCS I under the polyhedrin promoter and the 3CD gene fragment was inserted into MCS II under the p10 promoter. The resultant plasmid carrying two gene cassettes was designated pDual-P1-3CD (Figure 1). The recombinant baculoviruses were subsequently generated using Bac-to-Bac® system (Invitrogen) as described previously[29] and designated Bac-P1, Bac-3CD and Bac-P1-3CD based on the genes they encoded. The recombinant viruses were propagated, amplified, stored and titered according to standard procedures[30].
Figure 1 Construction of three recombinant baculovirus vectors using pFastBacTM DUAL plasmid as the backbone.
The gene fragments encoding P1 and 3CD were cloned separately into MCS I and II of pFastBacTM DUAL under the control of polyhedrin (Ppolh) and p10 promoters (Pp10), respectively. The resultant plasmids were designated pDual-P1 and pDual-3CD, respectively. The P1 and 3CD genes were cloned together into MCS I and II under the control of polyhedrin and p10 promoters. The resultant plasmid was designated pDual-P1-3CD.
SDS-PAGE and Western blot
To confirm the expression of recombinant P1 and VP1, the infected cells were harvested by low-speed centrifugation and then lysed by incubation in the lysis buffer (0.1% Triton X-100 in Tris-buffered saline) at
4 °C for 30 min. The proteins were resolved on 100 g/L gels as described previously[26] and stained by Commassie blue. For Western blot, the proteins were transferred onto nitrocellulose membranes according to standard procedures (Bio-Rad Laboratories). The transferred membrane was blocked with 50 g/L non-fat milk and probed by mouse anti-VP1 monoclonal antibody (MAb). The secondary antibody was goat anti-mouse IgG conjugated with alkaline phosphatase (Kirkegaard and Perry Laboratories). The membranes were developed with BCIP/NBT color developing reagent (Sigma).
Purification of VLP by ultracentrifugation
EV71 VLPs were produced by infecting Sf-9 cells with Bac-P1-3CD at a multiplicity of infection (MOI) of 10. Approximately 150 mL cells (1.5×106 cells/mL) were harvested at 2 d post-infection (dpi) by centrifugation
(1 000 r/min for 5 min) and were re-suspended in 10 mL TE buffer (0.01 mol/L Tris-HCl, 0.001 mol/L EDTA, pH 7.4). The cell suspension was supplemented with 1 mL of 10X HO buffer (0.01 mol/L Tris, 0.25 mol/L NaCl,
0.01 mo/L 2-mercaptoethanol) and 100 g/L NP-40, lysed by sonication for 3 min and centrifuged at 13 000 g (R20A2 rotor, CR22G, Hitachi) for 30 min. The supernatant was passed through a 0.22-μm filter to remove the debris and then ultracentrifuged at 27 000 g (P40ST rotor, CP100MX, Hitachi) for 4 h. The pellets were resuspended in TE buffer, sonicated for 30 s and loaded onto the discontinuous sucrose gradient (15%, 30% and 65% sucrose dissolved in TE buffer). After ultracentrifugation at 27 000 g (P40ST rotor) for 3 h, the milky white band between the interfaces of 15-35% sucrose was collected and layered on top of a 1.33 g cm-3 cesium chloride (CsCl) solution. After ultracentrifugation at 34 000 g (P40ST rotor) for 24 h, the bands collected from CsCl gradient were dialyzed against phosphate-buffered saline (pH 7.4) and were ultracentrifuged again at 34 000 g for 5 h. The pellets were resuspended in 50 μL pure water for SDS-PAGE, Western blot and transmission electron microscopy (TEM) analyses. The purities of the samples and the composition of VLPs were quantified by densitometry using Scion Image Shareware (Scion Corporation).
TEM and immunogold labeling
The VLPs within the insect cells were visualized under a transmission electron microscope (H-7500, Hitachi), prior to which ultrathin sectioning of cells was performed as described previously[26]. The particles after purification were adsorbed onto Formvar-coated copper grids, negatively stained by 20 g/L phosphotungstic acid (PTA) and examined by TEM. For immunogold labeling, purified particles were adhered onto copper grids and probed by mouse anti-VP1 MAb. The secondary antibody was goat anti-mouse IgG conjugated with 5 nm gold particles (Sigma). After washing of the unbound gold particles, the grids were stained by 20 g/L PTA and observed by TEM.
RESULTS
Expression of recombinant proteins in insect cells
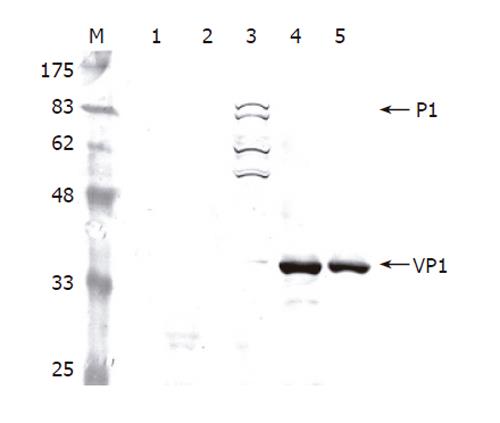
To determine whether the recombinant P1 and 3CD were successfully expressed by Bac-P1-3CD, Sf-9 cells were singly infected by wild-type AcMNPV, Bac-P1, and Bac-P1-3CD (MOI 10), or co-infected by Bac-P1 (MOI 5) and Bac-3CD (MOI 5). The cells were harvested at 3 dpi and lysed for Western blot analysis. As shown in Figure 2, the mock-infected and wild-type AcMNPV-infected cells did not express P1 (lanes 1 and 2), whereas the Bac-P1-infected cells expressed a protein with a molecular mass corresponding to 97 ku, indicating the successful expression of P1 (lane 3). Other P1-associated proteins migrating faster than P1 were also found (lane 3), suggesting a possible degradation of P1 by the cellular proteases, but this non-specific degradation did not yield VP1 (39 ku).
Figure 2 Western blot analysis of cell lysates infected by different viruses.
The Sf-9 cells were infected by the viruses at a total MOI of 10 and harvested at 3 dpi. The proteins were separated by SDS-PAGE, electrotransferred to a nitrocellulose membrane and probed using anti-VP1 MAb as the primary antibody. Lane 1: mock infection; Lane 2: wild-type baculovirus AcMNPV; Lane 3: Bac-P1; Lane 4: Bac-P1-3CD; Lane 5: Bac-P1 and Bac-3CD.
We also noted that no antibody specifically recognizing 3CD was available, hence the 3CD expression was detected indirectly by observing whether P1 was cleaved into VP1 upon co-expression of both proteins. Lanes 4 and 5 depict that either single infection by Bac-P1-3CD (lane 4) or co-infection by Bac-P1 and Bac-3CD (lane 5) led to complete cleavage of P1 into VP1, proving the successful co-expression of functional P1 and 3CD by both approaches.
Optimization of VP1 production
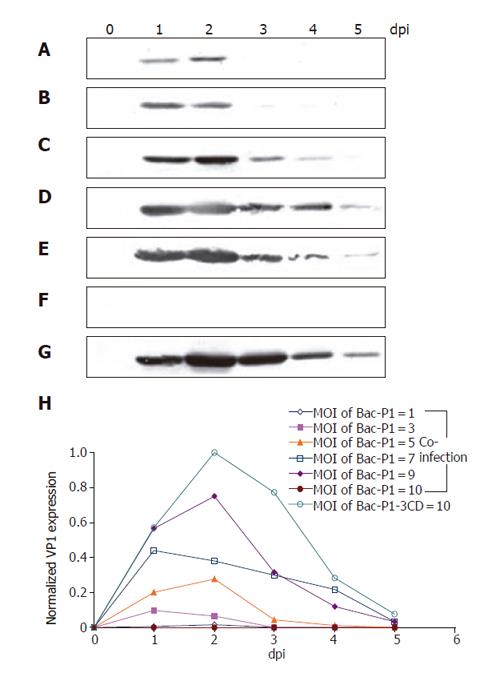
Since P1 relies on 3CD for cleavage and subsequent VLP assembly, delicately controlling the relative expression levels of P1 and 3CD is crucial for optimal VLP production. To address this issue, the cells were co-infected by Bac-P1 and Bac-3CD at MOI ratios of 1/9, 3/7, 5/5, 7/3, 9/1 and 10/0, or singly infected by Bac-P1-3CD at MOI 10 in order to compare and optimize the VP1 yield. Figures 3(A-G) show the time-course profiles of VP1 production, in which lanes 0-5 represent the samples harvested at 0, 1, 2, 3, 4 and 5 dpi, respectively. As shown, both single infection and co-infection approaches resulted in similar expression kinetics (except MOI 10/0), that is, the VP1 concentration rapidly culminated at 1-2 dpi, declined thereafter and virtually vanished at 5 dpi. The concentration peaked slightly earlier compared to those of many other proteins whose maximum concentration occurred at 3 dpi[27,31] probably because of proteolytic degradation by cellular proteases (data not shown).
Figure 3 Time course profiles of VP1 production under various infection conditions as analyzed by Western blot.
Insect cells were infected at a total MOI of 10 by single infection with Bac-P1-3CD (G), or co-infection at MOI ratios (Bac-P1/Bac-3CD) of 1/9 (b), 3/7 (B), 5/5 (C), 7/3 (D), 9/1 (E) and 10/0 (F). Lanes 0-5 represent the cell lysates collected at 0, 1, 2, 3, 4, and 5 dpi, respectively. The relative VP1 production levels under different conditions were quantified by scanning densitometry (H). The data represent the average values of duplicated experiments.
The production levels using different MOI combinations were also compared by scanning densitometry, by which all band intensities were normalized against the strongest band intensity (lane 2, Figure 5G). Figure 5H reveals that in the case of co-infection VP1 concentration increased with the MOI of Bac-P1 provided that the total MOI was fixed at 10. However, the maximum VP1 yield obtained from single infection (by Bac-P1-3CD at MOI 10) was the highest, being ≈30% and ≈120% greater than those obtained from co-infection at MOI 9/1 and 7/3, respectively.
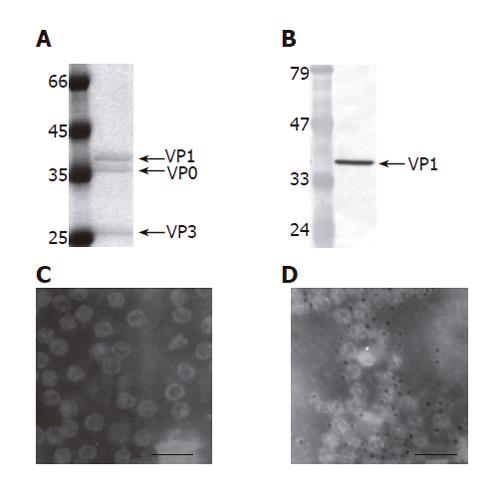
Figure 5 Purification and characterization of EV71 VLP (100 000 X magnification,).
The VLPs were produced by infecting 150 mL Sf-9 cells (1.5×106 cells/mL) with Bac-P1-3CD at MOI 10, harvested at 2 dpi and purified by ultracentrifugation. The purified VLPs were characterized by SDS-PAGE (A), Western blot (B), TEM examination (C) and immunogold labeling (D). The PAGE gel was stained by Commassie blue and scanned for densitometry analysis. The primary antibody used in the Western blot and immunogold labeling was anti-VP1 MAb. The size of the gold particles was 5 nm. Bar: 100 nm.
Formation of intracellular virus-like particle aggregates
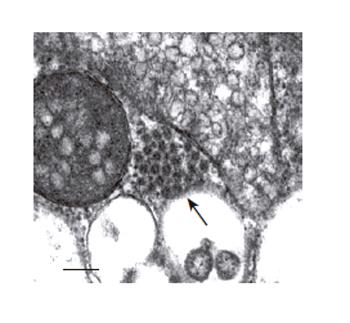
Since single infection with Bac-P1-3CD yielded the highest VP1 production, the formation of VLPs by single infection strategy was further examined. The cells were singly infected by Bac-P1-3CD at MOI 10 and subjected to ultrathin sectioning and TEM examination. As shown in Figure 4, clusters of virus-like particles were observed in the cytoplasm. These particles measured 25-27 nm in diameter and appeared icosahedral, thus resembling the authentic enterovirus in size and appearance. Such VLP aggregates were not observed in Sf-9 cells infected by Bac-P1 alone (data not shown), indicating that the particles were not the assembly products of individual structural proteins degraded from P1 precursor.
Figure 4 Electron microscopy examinations of EV71 VLP aggregates formed in cytoplasm of the Bac-P1-3CD-infected insect cells (100 000 X magnification).
The cells were infected at MOI 10, harvested at 3 dpi and ultrathin sectioned for TEM. The arrowhead indicates the VLP aggregate. Bar:100 nm.
Purification and characterization of EV 71 VLP
The subsequent VLP production was performed by infecting insect cells with Bac-P1-3CD (MOI 10). The particles were released from the infected cells, purified by ultracentrifugation and analyzed by SDS-PAGE (Figure 5A) and Western blot (Figure 5B). We observed that the purified sample contained three major proteins whose molecular masses corresponded to those of VP1 (39 ku), VP0 (36 ku) and VP3 (26 ku), and the purity of the sample was greater than 90% (Figure 5A). The molar concentrations of the three proteins as determined by scanning densitometry were roughly equal, agreeing with the notion that the molar ratios of VP0, VP1 and VP3 are identical in the enterovirus provirions[13]. Because no antibodies specifically recognizing VP0 and VP3 were available, the Western blot was performed using anti-VP1 antibody only, which further confirmed that the 39 ku protein was VP1. The purified particles were also subjected to TEM examination (Figure 5C), which showed that particles with size and morphology similar to those of the particles observed in vivo except the purified VLPs were dispersed. The immunogold labeling using anti-VP1 antibody further revealed gold particles surrounding the distinct particles (Figure 5D), indicating the exposure of VP1 on the outer surface. All these data confirmed that the Bac-P1-3CD infection successfully resulted in the formation of VLP comprising VP0, VP1 and VP3 and these particles could be purified by ultracentrifugation to near homogeneity.
The purified VLPs were also used as the standard to measure the specific yields of VLP. Approximately 10 mg of VLP was expressed per 109 Bac-P1-3CD-infected cells at MOI 10. This high expression level may contribute to future mass production and downstream purification.
DISCUSSION
The assembly pathway of EV71 has not been definitely identified, but the assembly model of poliovirus suggests that concerted cleavage of P1 by protease 3CD is required for capsid assembly. In this study, 3 recombinant baculoviruses were employed to express P1 and 3CD simultaneously in order to mimic the natural assembly process. By either single infection with Bac-P1-3CD or co-infection with Pac-P1 and Bac-3CD, successful processing of P1 into VP1 (Figure 1) was demonstrated, proving the successful expression of both P1 and functional 3CD. Alternative approach by co-expression of individual VP1, VP2, VP3 and VP4 proteins has been tested for VLP assembly, yet the yield was very low (personal communication). The comparison of these approaches suggests that VLP synthesis from the P1 precursor is a more favorable route, which agrees with the assembly pathway of poliovirus VLP[24].
The co-infection experiments further suggested that the amount of P1 required was larger than that of 3CD for VLP assembly as the co-infection at MOI 9/1 yielded considerably larger amounts of VP1 than MOI 5/5 or 1/9 (Figure 3). Intriguingly, we observed that single infection by Bac-P1-3CD was superior to co-infection by Bac-P1 and Bac-3CD with regard to VP1 production (Figure 3H). Since we lacked proper antibodies to detect 3CD, the relative expression levels of P1 and 3CD in the cells singly infected by Bac-P1-3CD remained unclear. However, since P1 expression was driven by polyhedrin promoter which was stronger than p10 promoter regulating the 3CD expression, one may surmise that differential expression of P1 and 3CD arose, namely, the P1 expression exceeded the 3CD expression. Another reason that may account for the higher production by single infection methodology is the VLP-producing cell population distribution as a result of the co-infection approach, whereby only the cells that were co-infected could produce VLP. Using the co-infection methodology, the probability of a cell to be infected by one or two types of viruses and the number of virus entering any given cells follow the modified Poisson distribution as predicted previously[32]. Hence, a population of cells that were singly infected by Bac-P1 or Bac-3CD arose, and no P1 processing or VLP assembly occurred in these cells. Even for the cells that were co-infected by both viruses, the number of infecting Bac-P1 and Bac-3CD within one cell might vary[32]. In cells where the number of Bac-P1 infection far more exceeded that of Bac-3CD infection, insufficient expression of 3CD and inadequate P1 cleavage could occur. On the contrary, in cells where the Bac-3CD infection considerably outnumbered the Bac-P1 infection, a drought in P1 supply could occur and lead to inadequate VLP assembly. Such heterogeneity could decrease the P1 processing and VLP assembly in many cells. In contrast, the infection of Bac-P1-3CD alone resulted in P1 processing in all cells, leading to a higher VP1 yield.
Despite the lower yield, the significance of co-infection strategy should not be ignored because it offers a new dimension to manipulate the composition of VLPs. For instance, the cells may be co-infected with Bac-P1-3CD and a new recombinant baculovirus expressing VP1 derived from a different EV71 strain (e.g., the prototype BrCr strain). The phenotypic mixing may allow the incorporation of VP1 from BrCr strain into the VLP, thus generating chimeric, multivalent VLPs and broadening the spectrum of protection.
In addition to the higher expression level, single infection strategy resulted in the formation of VLP morphologically indistinguishable from the authentic viruses. Within the cells, clusters of virus-like particles resembling those observed in EV71-infected human rhabdomyosarcoma (RD) cells[26] and in enterovirus-infected skeletal muscle cells[33] were observed. The in vivo formation of particle aggregates was not previously reported by other researchers studying the poliovirus VLPs[23,25], but was recently noted in BHK-38 cells infected by foot-and-mouth disease virus[34], a relatively genetically distant picornavirus. To date, the biological significance of the particle aggregation remains to be explored, but the resemblance in particle morphology and the aggregation patterns between the VLP and the authentic enterovirus suggests that EV71 VLP adopts an assembly pathway in insect cells in a way similar to the authentic viruses do in their natural host cells. Despite the aggregation within cells, the VLP could be dispersed in solution and purified through CsCl gradient ultracentrifugation to near homogeneity. The purified VLPs comprised VP0, VP3 and VP1 of roughly equal molar ratios, a result consistent with previous findings that poliovirus provirions are composed of 60 copies each of VP1, VP3 and VP0[13]. This implied the similarity between the VLP and the authentic virus in composition. Notably, in insect cells VP0 did not appear to undergo further cleavage into VP2 and VP4, a maturation process associated with the acquisition of infectivity and the increase in particle stability[35]. The lack of further processing is reasonable as the natural maturation process takes place after the RNA encapsidation, yet in this expression system, no RNA packaging into the central cavity occurred.
Nonetheless, after purification, the size and morphology of VLPs appeared indistinguishable from those of poliovirus VLP. More importantly, the immunogold labeling confirmed the presence of VP1 epitopes on the VLP surface. These data suggest that the VLPs contain the important antigenic sites essential for eliciting the immune responses. This is particularly important because neutralizing epitopes are most densely clustered on VP1 and many of them consist of amino acids from different domains or from different polypeptides[36,37], that is, many of them are conformation-dependent. In this regard, the use of VLP as a vaccine candidate against EV71 infection may be particularly promising. In recent years, VLP-based vaccines have been demonstrated to not only elicit neutralizing antibodies, but also provoke potent cytotoxic and helper T-lymphocyte responses and have been shown to confer protection from virus challenge in the animal models[38-40]. Furthermore, papillomavirus-like particles have been shown to induce acute activation of dendritic cells[16]. Other VLPs that have been evaluated in pre-clinical studies for their potentials as vaccines include JC virus, human immunodeficiency virus, rotavirus and parvovirus[41]. Moreover, the humoral, mucosal and cellular immune responses to Norwalk VLP in humans have been reported[42], while VLP vaccines against human papillomavirus have entered into phase II clinical trials and promising data have been presented[43]. All these further justify the development of VLP vaccines.
Since EV71 has caused seasonal morbidity and mortality in children with increasing frequency in recent years, vaccine development has become a top priority among all control strategies. Although other vaccine types, such as inactivated virus and VP1 subunit vaccines, have been tested[44], only the inactivated virus elicited strong immune responses, again confirming the importance of preserving epitope conformation. However, further assessment of safety and efficacy of the inactivated virus vaccine is required. In this study, we have successfully demonstrated the formation and purification of EV71 VLP via co-expression of P1 and 3CD and a reasonable production yield (≈10 mg/109 cells) was achieved, thus offering an attractive alternative to the inactivated virus vaccine. At present, the immunological evaluation has been impeded due to the difficulty in obtaining sufficient amounts of pure VLP because the recovery yield of VLP by ultracentrifugation was very low. The poor recovery yield may be attributed to the impaired VLP stability as empty capsids are less stable than mature virions[45]. The particle stability may be enhanced by the addition of a stabilizer (e.g., pirodavir, disoxaril or R 78206) that binds the pocket underneath the canyon of the particle[46]. The feasibility of enhancing VLP integrity by stabilizers is being evaluated. Furthermore, the development of a more efficient chromatography-based purification scheme is necessary for process scale-up and is underway.