Published online Feb 14, 2006. doi: 10.3748/wjg.v12.i6.868
Revised: July 20, 2005
Accepted: July 28, 2005
Published online: February 14, 2006
AIM: To investigate the relationship between the chemotherapeutic drug efficacy and the expression of P-glycoprotein (PGP) and p53 protein in advanced hepatocellular carcinoma (HCC).
METHODS: The study was conducted on 41 patients with advanced HCC who were treated by repeated arterial infusion chemotherapy. Biopsy specimens from the tumor were collected before the start of treatment in all the patients, and the specimens were stored frozen until immunohistochemical staining, which was performed after the start of treatment, to detect PGP and p53 protein expressions. Twenty of the forty-one patients were treated with an anthracycline drug (epirubicin hydrochloride; anthracycline group), and the remaining 21 were treated with a non-anthracycline drug (mitoxantrone hydrochloride in 11 patients and carboplatin in 10 patients; non-anthracycline group). The relationship between the chemotherapeutic efficacy and the results of immunostaining were compared between the two groups.
RESULTS: Before the start of the treatment, PGP-positive rate was 90.2% (strongly-positive, 36.6%) and p53 protein-positive rate was 34.1% (strongly-positive, 19.5%). In the anthracycline group, the response rate was 40.0%. The number of patients showing poor response to the treatment was significantly larger in the patients with strongly positive PGP expression (P = 0.005), and their prognoses were poor (P = 0.001). In the non-anthracycline group, the response rate was 42.9%, and there was no significant relationship between the chemotherapeutic drug efficacy and the PGP or p53 protein expression. When only the data from the 11 patients treated with anthraquinone drug, mitoxantrone, were analyzed, however, the number of patients who showed poor response to treatment was significantly higher among the p53-positive patients (P = 0.012), irrespective of the survival outcome.
CONCLUSION: The chemotherapeutic efficacy with an anthracycline drug for advanced HCC can be predicted by immunohistochemical analysis of PGP expression. Similarly, immunostaining to evaluate p53 protein may be useful to predict the response in patients treated with an anthraquinone drug.
- Citation: Akimoto M, Yoshikawa M, Ebara M, Sato T, Fukuda H, Kondo F, Saisho H. Relationship between therapeutic efficacy of arterial infusion chemotherapy and expression of P-glycoprotein and p53 protein in advanced hepatocellular carcinoma. World J Gastroenterol 2006; 12(6): 868-873
- URL: https://www.wjgnet.com/1007-9327/full/v12/i6/868.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i6.868
Transcatheter hepatic arterial embolization (TAE) is widely employed for the treatment of inoperable advanced hepatocellular carcinoma (HCC). However, TAE is unsuitable for the cases with a tumor embolus in the main trunk or the first branch of the portal vein, or for those with severe hepatic injury. For these cases, repeated arterial infusion chemotherapy (RAIC) with a reservoir may be useful, although it is still difficult, at present, to assert that RAIC would provide satisfactory therapeutic results.
Drug resistance is a serious problem in the treatment of HCC, and is mediated by various mechanisms, including those mediated by P-glycoprotein (PGP) and p53 protein expressions. PGP is known to act as an ATP-dependent pump that facilitates transport of substances, including anticancer drugs, from within to outside the cells, facilitating the development of multidrug resistance, including that to anthracyclines[1]. Moreover, resistance to apoptosis conferred by p53 protein expression is also thought to be a potential factor in the development of multidrug resistance in cancer cells[2]. It has been reported that a mutant-type p53 protein may emerge in various types of cancers due to gene deletion, recombination, or point mutations. This mutant type of p53 protein has been reported to have a considerably longer half life as compared with its wild-type counterpart[3], and it is thought to act as a dominant-negative inhibitor against the wild-type protein, influencing the anti-tumor effects of anticancer drugs[4].
While PGP and p53 protein expressions have been reported in HCC, mainly from the examination of surgically resected specimens, only a few reports have discussed the relationship between the efficacy of chemotherapeutic agents and the expression of PGP in cases of HCC[5,6]; the relationship between the outcome of arterial infusion chemotherapy and the expression of PGP and p53 protein remains particularly obscure. In this study, immunohistochemical analysis was conducted on frozen biopsy specimens obtained from patients with advanced HCC before the treatment to evaluate PGP and p53 protein expressions, and the therapeutic outcomes of RAIC in relation to the expression of these proteins were compared by the type of drugs that were administered.
One hundred and thirty patients were diagnosed to have an unresectable advanced HCC and treated by RAIC at the Department of Medicine and Clinical Oncology, School of Medicine, Chiba University, and its affiliated institutes between April 1995 and June 1997. We could obtain tumor biopsy specimens before the initiation of RAIC from 41 of these patients, and stored these specimens at -80 °C until the assay. Immunohistochemical staining for PGP and p53 expressions was performed on these specimens and the results were analyzed in relation to the therapeutic outcome in the patients.
Immediately before the start of RAIC, two or three biopsy specimens were obtained percutaneously using a 21G biopsy needle under ultrasound guidance from 41 patients. The tissue specimens were embedded in OCT compound and immediately stored at -80 °C.
For the immunohistochemical staining, frozen sections (5 µm thick) were prepared and fixed with acetone for 5 min. After pretreatment to prevent non-specific staining, the sections were allowed to react overnight at 4 °C with mdr (Ab-1, diluted 1:250) (Oncogene Research Products, Cambridge, MA, USA) as the primary antibody for PGP. For p53 protein staining, the sections were allowed to react at room temperature for 1 h with DO-7 (diluted 1:40, DAKO, Denmark). The sections were then successively treated with the secondary antibody, and then stained by the avidin-biotinylated peroxidase complex method for PGP, and the streptavidin-biotin method for p53 protein. Color development was conducted by DAB staining, and nuclear staining was conducted with Mayer’s hematoxylin for PGP and methyl green for the p53 protein.
Light-microscopic examinations of the stained sections were conducted to detect positive staining for PGP on the cytoplasmic membranes or for p53 protein within the nuclei of the cells in the cancerous areas. At least 300 cells were examined, and the staining intensity was classified based on the percentage of positively stained cells as follows: negative (0-5%); weakly positive (6%-30%); and strongly positive (31% or more).
A 6F indwelling catheter (Anthron PU®; Toray, Tokyo) was introduced percutaneously through the left subaxillary artery (16 patients) or right femoral artery (25 patients) up to the level of the proper hepatic artery or common hepatic artery. The catheter was connected at the other end to a subcutaneously implanted reservoir (Port-A-Cath®, Pharmacia Deltec, St. Paul, MN, USA; or Cersite®, Toray, Tokyo)[7,8].
An anticancer drug was administered over a period of 15 min once in every 4 wk at the outpatient clinic, after first confirming the patency of the catheter by fluoroscopy. Twenty, eleven, and ten of the forty-one patients received epirubicin hydrochloride, mitoxantrone hydrochloride, and carboplatin, respectively. The doses for each administration were set as follows: 70 mg/body surface area for epirubicin hydrochloride, 12 mg/body surface area for mitoxantrone hydrochloride, and
300 mg/body surface area for carboplatin. No other therapy was administered concomitantly in any of the patients. All the patients had underlying liver cirrhosis, and the diagnosis of HCC was confirmed histopathologically in all of them. Fifteen of the forty-one patients had received TAE prior to the RAIC with epirubicin hydrochloride at 40-70 mg/body surface area, while in the remaining 26 patients, RAIC was administered as the initial therapy. Informed consent was obtained from all the patients prior to tissue biopsy and RAIC. In this study, these patients were divided into anthracycline group (20 patients) treated with the anthracycline drugs, epirubicin hydrochloride and non-anthracycline group (remaining 21 patients) treated with the other drugs. No significant differences in the background characteristics were observed between the anthracycline group and the non-anthracycline group (Table 1). The therapeutic efficacies of the drugs were evaluated according to WHO guidelines. The anticancer drug administration was discontinued in the event of detection of any serious side effects, such as leucopenia, thrombocytopenia, or hepatic dysfunction. Observation of the patients was continued until September 30, 2003, by which date, all of the patients died.
| Anthracycline group | Non-anthracycline group | ||
| Age (yr) | Mean | 60.5 ± 7.2 | 62.1 ± 8.8 |
| Gender1 | Male | 18 | 19 |
| Female | 2 | 2 | |
| Tumor stage12 | III | 1 | 1 |
| IV | 19 | 20 | |
| Child’s class1 | A | 12 | 11 |
| B | 6 | 9 | |
| C | 2 | 1 | |
| Hepatitis virus1 | B( - ) C( + ) | 15 | 14 |
| B( + ) C( + ) | 3 | 5 | |
| B( + ) C( - ) | 0 | 1 | |
| B( - ) C( - ) | 2 | 1 | |
| Chemotherapy13 | ( - ) | 13 | 13 |
| ( + ) | 7 | 8 | |
| HCC differentiation1 | Well | 1 | 2 |
| Moderately | 14 | 15 | |
| Poorly | 5 | 4 |
The data were statistically analyzed using χ2 test or Mann–Whitney’s U-test. Differences at a P value of 5% or lower were considered to be statistically significant. The survival rate from the start of the RAIC was calculated by the Kaplan-Meier method and analyzed using the log rank test.
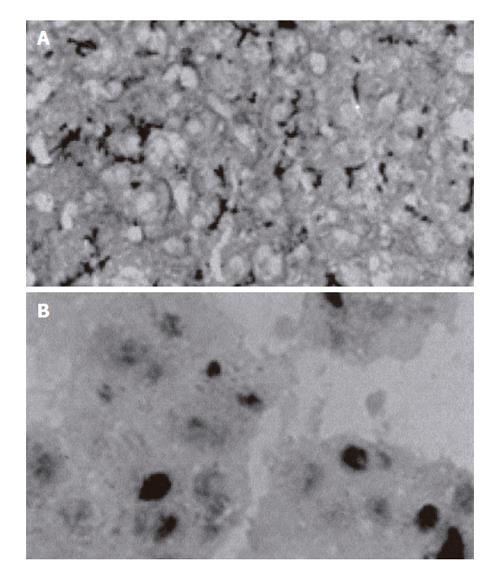
Strongly-positive, weakly-positive, and negative PGP expressions were observed in 15 (36.6%), 22 (53.7%), and 4 patients (9.8%), respectively. Strongly-positive, weakly-positive, and negative p53 protein expressions were observed in 8 (19.5%), 6 (14.6%), and 27 patients (65.9%), respectively. Sections showing strong positivity for PGP and p53 protein expressions on immunohistochemical staining are shown in Figures 1A and B, respectively.
Of the 15 patients who had received TAE with anthracycline, 9 (60%) and 6 (40%) were found to be strongly and weakly positive for PGP, respectively, and none of the cases showed negative staining for PGP. Of the 26 patients who had not undergone TAE prior to the RAIC, 6 (23.1%), 16 (61.5%), and 4 (15.4%) patients were found to have strongly positive, weakly positive, and negative PGP expressions, respectively. Thus, the number of cases showing strong positivity for PGP expression was significantly higher among those who had a previous history of treatment with an anthracycline drug than those without such history (P = 0.036). The percentage of cases showing p53 protein expression did not differ significantly among the patients with or without a history of treatment with an anthracycline drug.
No significant relationship was observed between the expression of PGP and that of the p53 protein (Table 2).
| PGP positivity | |||
| Positivity | Strongly-positive | Weakly-positive | Negative |
| p53 strongly-positive | 5 | 2 | 1 |
| p53 weakly-positive | 2 | 4 | 0 |
| p53 negative | 8 | 16 | 3 |
| Not significant (χ2) | |||
Of the 41 patients, the 20 belonging to the anthracycline group received average arterial infusions of 11.4 ± 8.1 (range, 3-34) times during a mean follow-up period of 14.5 (range, 3-43) mo; 95%CI = 0.00-30.3. Of these 20 patients, 2, 6, 7, and 5 patients showed CR, PR, NC, and PD, respectively, yielding a response rate of 40.0% (95%CI = 18.5-61.5). In the 21 patients belonging to the non-anthracycline group, the mean number of arterial infusions and the mean duration of follow-up were 13.3 ± 9.5 (range, 3-38) times and 18.7 (range, 3-78) mo; 95%CI = 8.1-29.4, respectively. In this group, 3, 6, 8, and 4 patients showed CR, PR, NC, and PD, respectively, yielding a response rate of 42.9% (95%CI = 21.7-64.0).
The expression rate of PGP and p53 protein did not differ significantly between the anthracycline group and the non-anthracycline group (Table 3). Since the positive rate of PGP expression was very high in cases of HCC, the patients were further divided into those showing strongly positive expression (positive group) and those showing weakly positive or negative expression (non-positive group); a similar classification of the patients was conducted for p53 protein expression, into those showing strongly or weakly positive expression (positive group) and those showing negative expression (non-positive group). As for the relationship of the therapeutic outcome to PGP expression in the anthracycline group, the number of non-responders was significantly higher in the positive group (P = 0.005); on the other hand, no such difference regarding PGP expression was found in the non-anthracycline group. In regard to the correlation of the therapeutic outcome with the expression of p53 protein, no significant differences between the positive and non-positive patients were found in either the anthracycline group or in the non-anthracycline group. But when only the data of the 11 mitoxantrone-treated patients of the non-anthracycline group were analyzed, 2 and 4 patients in the non-p53-positive group showed CR and PR, respectively, while 4 and 1 patients in the p53-positive group showed NC and PD, respectively, demonstrating the significant correlation between therapeutic outcome and the expression status of the p53 protein (P = 0.012). On the other hand, in the remaining 10 carboplatin-treated patients in this group, 1, 2, 4, and 2 patients in the non-p53-positive group showed CR, PR, NC, and PD, respectively, and the remaining one p53-positive patient showed PD, revealing the absence of any significant correlation between the therapeutic outcome and the p53 protein expression status.
| Positivity | Anthracycline group (n = 20) | Response rate (%) (95%CI) | Non-anthracycline group (n = 21) | Response rate (%) (95%CI) | ||||||
| CR | PR | NC | PD | CR | PR | NC | PD | |||
| PGP12 | ||||||||||
| Non-positive group | 2 | 6 | 3 | 0 | 72.7 (46.4-99.1)b | 3 | 5 | 5 | 2 | 53.3 (28.1-78.6) |
| Positive group | 0 | 0 | 4 | 5 | 0.0 (0.0-0.0) | 0 | 1 | 3 | 2 | 16.7 (-13.2-46.5) |
| p5313 | ||||||||||
| Non-positive group | 2 | 4 | 2 | 4 | 50.0 (21.7-78.3) | 3 | 6 | 6 | 2 | 52.9 (29.2-76.7) |
| Positive group | 0 | 2 | 5 | 1 | 25.0 (-5.0-55.0) | 0 | 0 | 2 | 2 | 0.0 (0.0-0.0) |
The relationships between the positive rates for PGP or p53 protein and the survival rate are shown in Table 4. With regard to the PGP expression status in the anthracycline group, the survival rate was significantly lower in the positive group compared to non-positive group (P = 0.001); on the other hand, in the non-anthracycline group, no significant difference was observed between patients in the positive and non-positive group. As for the p53 protein expression status, no significant correlation was observed between the expression and the survival rate in either the anthracycline or the non-anthracycline group, including the 11 mitoxantrone-treated patients.
| Positivity | Number of patients | Survival rates (%) | Median survival | 95%CI | ||
| 12 mo | 24 mo | 36 mo | time (mo) | |||
| Anthracycline group | ||||||
| PGP | ||||||
| Non-positive | 11 | 90.9 | 54.6 | 9.1 | 26.5 | 15.8-37.2b |
| Positive | 9 | 11.1 | 0.0 | 0.0 | 6.6 | 6.4-6.8 |
| P53 | ||||||
| Non-positive | 12 | 58.3 | 41.7 | 8.3 | 18.3 | 0.0-41.4 |
| Positive | 8 | 50.0 | 12.5 | 0.0 | 9.7 | 0.0-20.7 |
| Non-anthracycline group | ||||||
| PGP | ||||||
| Non-positive | 15 | 80.0 | 53.3 | 33.3 | 24.5 | 13.3-35.8 |
| Positive | 6 | 50.0 | 16.7 | 16.7 | 9.3 | 0.0-21.2 |
| P53 | ||||||
| Non-positive | 15 | 80.0 | 46.7 | 33.3 | 18.7 | 5.7-31.8 |
| Positive | 6 | 50.0 | 33.3 | 16.7 | 9.3 | 0.0-25.2 |
In conventional studies, the PGP-positive rate has been determined, mainly based on the examination of surgically resected specimens, to range from 52% to 92%[5,9]. It has also been suggested that the expression of PGP decreases with decreasing degree of differentiation[10]. In the present study, our patients had a relatively high PGP-positive rate of 90.2%, when weakly positive cases were also included. It has been suggested that PGP expression might be induced by previously administered anthracycline chemotherapy in some cases[11], and that following therapy, only the PGP-positive cells survived, resulting in the high overall positivity rate. However, further studies are required because the study population was small and we could not perform biopsies of the tumors for the duration of chemotherapy or after chemotherapy. On the other hand, the p53-positivity rate in patients with HCC has been reported to range from 12.5% to 51.2%[6,10]. Consistent with these reports, the p53-positivity rate in our present study was 34.1%.
As to the relationship between the p53 tumor suppressor gene expression and the MDR1 gene expression, while some reported that the wild-type p53 protein represses the MDR1 promoter and the mutant-type p53 protein stimulates it, other investigators have suggested that the wild-type p53 stimulates the expression of this promoter[12,13]. In our patients, no significant correlation was observed between the expression of PGP and that of the mutant-type p53 protein, suggesting that mutation of p53 may not regulate the expression of PGP, consistent with recently reported studies[6,9,10]. In the present study, p53 protein was examined only by immunostaining using DO-7 which indicates the expressions of p53 protein variants. Therefore, a relationship between wild-type p53 protein and expressions of PGP could not be identified.
A relationship between PGP expression and the therapeutic efficacy of chemotherapeutic agents has been reported in cases of osteosarcoma and neuroblastoma[14,15]. On the other hand, while no significant correlation was observed between the therapeutic outcome and the expression of PGP in 13 patients with HCC treated with hepatic arterial infusion of doxorubicin[5], a significant correlation between the two was observed in 25 patients with advanced HCC treated by oral or intravenous administration of VP-16 or doxorubicin[6]. In the present study on RAIC, the expression of PGP was significantly correlated with the therapeutic outcome and prognosis of the patients with advanced HCC, but only in the group treated with an anthracycline drug. These results, obtained from actually treated clinical cases of HCC, provide important evidence supporting the involvement of PGP in anthracycline resistance which was demonstrated in basic scientific studies[1]. In addition, resistance mediated by alteration of nuclear topoisomerase, rather than PGP, has also been reported in patients receiving anthracycline drugs[16], necessitating further studies on this issue. No significant correlation was found in this study between the expression of PGP and the therapeutic outcome or prognosis in patients treated with non-anthracycline drugs, such as an anthraquinone or a platinum-based drug. It is speculated that canalicular multispecific organic anion transporter (cMOAT) is involved in the excretion of cisplatin, and over-expression of cMOAT has been observed in an HCC cell line[17].
As for the p53 protein, expression of a mutant-type of p53 protein has been reported to be associated with a poor prognosis[10,18], and it has been suggested that apoptosis induced by anti-cancer drugs, such as doxorubicin, mitoxantrone, cisplatin and bleomycin, is mediated, at least in part, by p53-dependent stimulation of the CD95 receptor/ligand system[19,20]. However, no relationship between the chemotherapeutic drug efficacy and expression of the p53 protein has been reported until now in patients with HCC. In our present study, expression of p53 protein was not correlated with the chemotherapeutic drug efficacy or prognosis in patients treated with the anthracycline drug, suggesting a lesser importance of the expression of a mutant-type p53 protein, as compared with that of PGP, in the evaluation of the sensitivity of advanced HCC to anthracyclines. As for the anthraquinones among the non-anthracycline drugs, a fundamental study in a mitoxantrone-resistant cell line revealed that the expression of wild-type p53 protein induced cellular apoptosis[21]; on the other hand, in relation to platinum-based drugs, expression of an abnormal type of p53 protein was reported to be associated with resistance to chemotherapy in cases of lung cancer[22]. In our patients, treated with an anthraquinone, a significant correlation was observed between the expression of the p53 protein and the chemotherapeutic drug efficacy, but not the prognosis of patients with advanced HCC. On the other hand, no such correlation of the p53 expression was observed with either the chemotherapeutic drug efficacy or the prognosis in HCC patients treated with the platinum-based drug, carboplatin. Although further studies are required, these results indicate the possibility that evaluation of the expression of a mutant-type p53 protein may be useful for predicting the sensitivity of HCC to anthraquinone drugs.
In conclusion, our study demonstrates the existence of a correlation between the outcome of arterial infusion chemotherapy and the expression of PGP and p53 protein in patients with advanced HCC. Therefore, it is suggested that immunostaining for PGP may be useful for predicting the effectiveness of RAIC in cases treated with an anthracycline drug, and that p53 protein expression may be useful for predicting the outcome in cases treated with an anthraquinone drug.
The staff of the Department of Medicine and Clinical Oncology and Department of Molecular Pathology, School of Medicine, Chiba University generously supported the completion of this study.
S- Editor Kumar M and Guo SY L- Editor Elsevier HK E- Editor Kong LH
| 1. | Leveille-Webster CR, Arias IM. The biology of the P-glycoproteins. J Membr Biol. 1995;143:89-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Kataoka S, Naito M, Tomida A, Tsuruo T. Resistance to antitumor agent-induced apoptosis in a mutant of human myeloid leukemia U937 cells. Exp Cell Res. 1994;215:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Harris CC, Hollstein M. Clinical implications of the p53 tumor-suppressor gene. N Engl J Med. 1993;329:1318-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 943] [Cited by in RCA: 930] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 4. | Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2022] [Cited by in RCA: 2066] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 5. | Itsubo M, Ishikawa T, Toda G, Tanaka M. Immunohistochemical study of expression and cellular localization of the multidrug resistance gene product P-glycoprotein in primary liver carcinoma. Cancer. 1994;73:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Chou YY, Cheng AL, Hsu HC. Expression of P-glycoprotein and p53 in advanced hepatocellular carcinoma treated by single agent chemotherapy: clinical correlation. J Gastroenterol Hepatol. 1997;12:569-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Yoshikawa M, Ebara M, Nakano T, Minoyama A, Sugiura N, Ohto M. Percutaneous transaxillary catheter insertion for hepatic artery infusion chemotherapy. AJR Am J Roentgenol. 1992;158:885-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Yoshikawa M, Ebara M, Saisho H, Hatano R, Morimoto N, Sanada M, Fukuda H, Sugiura N, Ohto M. Long-term outcome of percutaneously implanted port and catheter for hepatic arterial chemoinfusion. J Int Radiol. 1997;12:137-142. |
| 9. | Ng IO, Liu CL, Fan ST, Ng M. Expression of P-glycoprotein in hepatocellular carcinoma. A determinant of chemotherapy response. Am J Clin Pathol. 2000;113:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Nakano A, Watanabe N, Nishizaki Y, Takashimizu S, Matsuzaki S. Immunohistochemical studies on the expression of P-glycoprotein and p53 in relation to histological differentiation and cell proliferation in hepatocellular carcinoma. Hepatol Res. 2003;25:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Goldstein LJ, Galski H, Fojo A, Willingham M, Lai SL, Gazdar A, Pirker R, Green A, Crist W, Brodeur GM. Expression of a multidrug resistance gene in human cancers. J Natl Cancer Inst. 1989;81:116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 898] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 12. | Chin KV, Ueda K, Pastan I, Gottesman MM. Modulation of activity of the promoter of the human MDR1 gene by Ras and p53. Science. 1992;255:459-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 565] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 13. | Goldsmith ME, Gudas JM, Schneider E, Cowan KH. Wild type p53 stimulates expression from the human multidrug resistance promoter in a p53-negative cell line. J Biol Chem. 1995;270:1894-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Baldini N, Scotlandi K, Barbanti-Brodano G, Manara MC, Maurici D, Bacci G, Bertoni F, Picci P, Sottili S, Campanacci M. Expression of P-glycoprotein in high-grade osteosarcomas in relation to clinical outcome. N Engl J Med. 1995;333:1380-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 266] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 15. | Chan HS, Haddad G, Thorner PS, DeBoer G, Lin YP, Ondrusek N, Yeger H, Ling V. P-glycoprotein expression as a predictor of the outcome of therapy for neuroblastoma. N Engl J Med. 1991;325:1608-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 305] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Cole SP, Chanda ER, Dicke FP, Gerlach JH, Mirski SE. Non-P-glycoprotein-mediated multidrug resistance in a small cell lung cancer cell line: evidence for decreased susceptibility to drug-induced DNA damage and reduced levels of topoisomerase II. Cancer Res. 1991;51:3345-3352. [PubMed] |
| 17. | Minemura M, Tanimura H, Tabor E. Overexpression of multidrug resistance genes MDR1 and cMOAT in human hepatocellular carcinoma and hepatoblastoma cell lines. Int J Oncol. 1999;15:559-563. [PubMed] |
| 18. | Nishio M, Koshikawa T, Kuroishi T, Suyama M, Uchida K, Takagi Y, Washimi O, Sugiura T, Ariyoshi Y, Takahashi T. Prognostic significance of abnormal p53 accumulation in primary, resected non-small-cell lung cancers. J Clin Oncol. 1996;14:497-502. [PubMed] |
| 19. | Muller M, Strand S, Hug H, Heinemann EM, Walczak H, Hofmann WJ, Stremmel W, Krammer PH, Galle PR. Drug-induced apoptosis in hepatoma cells is mediated by the CD95 (APO-1/Fas) receptor/ligand system and involves activation of wild-type p53. J Clin Invest. 1997;99:403-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 544] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 20. | Muller M, Wilder S, Bannasch D, Israeli D, Lehlbach K, Li-Weber M, Friedman SL, Galle PR, Stremmel W, Oren M. p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J Exp Med. 1998;188:2033-2045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 635] [Cited by in RCA: 606] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 21. | Seth P, Katayose D, Li Z, Kim M, Wersto R, Craig C, Shanmugam N, Ohri E, Mudahar B, Rakkar AN. A recombinant adenovirus expressing wild type p53 induces apoptosis in drug-resistant human breast cancer cells: a gene therapy approach for drug-resistant cancers. Cancer Gene Ther. 1997;4:383-390. [PubMed] |
| 22. | Rusch V, Klimstra D, Venkatraman E, Oliver J, Martini N, Gralla R, Kris M, Dmitrovsky E. Aberrant p53 expression predicts clinical resistance to cisplatin-based chemotherapy in locally advanced non-small cell lung cancer. Cancer Res. 1995;55:5038-5042. [PubMed] |