Published online Feb 7, 2006. doi: 10.3748/wjg.v12.i5.815
Revised: August 1, 2005
Accepted: August 3, 2005
Published online: February 7, 2006
The term gastrointestinal stromal tumors (GISTs) is defined diagnostically as the main group of mesenchymal tumors with spindle or epithelioid cells arising from the wall of the gastrointestinal tract with immunohistochemical reactivity for CD117 antibody. Previous studies revealed that cells in GISTs express a growth factor receptor with tyrosine kinase activity (termed c-kit), which is the product of the c-kit proto-oncogene. The most specific and practical diagnostic criteria for GISTs are: immunohistochemically determined c-kit (CD117) expression; mitotic score; and tumor size. A small GIST concomitant with early gastric cancer is rarely encountered clinically. Herein we have reported a case of a 1.1-cm GIST detected by esophagogastroduodenoscopy concomitant with a IIc type of early gastric cancer (signet ring cell type). It was detected during a routine physical health examination. To our knowledge, this is the first report of a small GIST concomitant with a signet ring cell type of early gastric cancer.
- Citation: Lin YL, Tzeng JE, Wei CK, Lin CW. Small gastrointestinal stromal tumor concomitant with early gastric cancer: A case report. World J Gastroenterol 2006; 12(5): 815-817
- URL: https://www.wjgnet.com/1007-9327/full/v12/i5/815.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i5.815
Gastrointestinal stromal tumors (GISTs) are malignant or potentially malignant tumors and are considered to have a specific molecular pathogenesis. They are distinguished from other mesenchymal tumors by optimal immunostaining for CD117 and a prognostic classification is based on tumor size, mitotic score, and MIB-1 grade[1]. Gain-of-function mutations of the c-kit gene and immunoreactivity of the c-kit protein (CD117) in many GISTs support the idea that GISTs are a biologically distinct entity. We have reported a case of a small GIST concomitant with a IIc type of early gastric cancer (signet ring cell type) and provided a literature review.
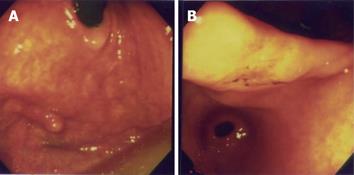
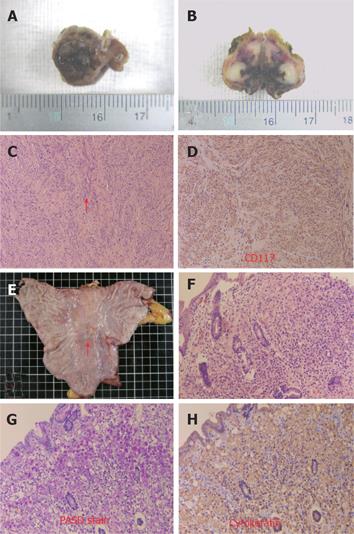
A 70-year-old yellow race female came to our hospital for a physical health examination in November 2003. She had no past history of epigastralgia or peptic ulcer. Esophagogastroduodenoscopy (EGD) showed one approximately 0.4-cm sessile polyp with a smooth surface at the right upper posterior wall of the gastric fundus (Figure 1A). Biopsy was taken and three specimens were acquired. Histologically, one of the three specimens showed whorling bundles of spindle cells with mitosis. Only chronic gastritis was found in the other two specimens. A GIST was suspected. EGD simultaneously detected one approximately 1.7 cm × 1.4 cm, depressed, flat white-red based lesion with oozing hemorrhages at the gastric angle. The lesion looked like a IIc type of early gastric cancer (Figure 1B). Biopsy was carried out and 13 specimens were obtained. Pathological sections demonstrated an ill-defined tumor with signet ring cells within the gastric mucosa. A poorly differentiated adenocarcinoma was diagnosed and Helicobacter microorganisms were found. An imaging study with abdominal computed tomography demonstrated no remarkable mass lesion at the posterior wall of the gastric fundus and gastric angle. There was no fluid collection, and no mass lesion or lymph node was found in the intraperitoneal cavity, liver, kidneys, and other organs. Owing to its malignant nature, surgical intervention was performed one week later. Grossly, we observed a 1.1 cm × 0.8 cm × 0.7 cm fundal mass protruding from the inner muscularis propria to the mucosa (Figures 2A and 2B). Histologically, the gastric fundal tumor demonstrated whorling sheets of spindle cells which stained positively for CD117, CD34, NSE, S-100 protein, and actin-851 antibodies after immunohistochemical (IHC) staining (Figures 2C and 2D). GIST, with combined smooth muscle-neural differentiation, was diagnosed. The gastric angular tumor showed a residual adenocarcinoma of the signet ring cell type within the mucosa (Figures 2E and 2H). The postoperative period of the patient was uneventful, and she was discharged one week later. No evidence of tumor recurrence was found after 14-months of follow-up.
GIST concomitant with early gastric cancer has rarely been reported. To the best of our knowledge, a small GIST concomitant with a signet ring cell type of early gastric cancer has never been reported. Although Japanese investigators reported some cases of gastric leiomyoblastoma associated with gastric cancer between 1971 and 2000, CD-117 immunohistochemical staining was not confirmed in those cases. Our 1.1-cm GIST detected by EGD was a small one. The origin of a GIST concomitant with an early gastric adenocarcinoma is unclear. This small GIST showed a positive microscopic finding of H pylori microorganisms and a positive CLO test (biopsy urease test) for H pylori infections. H pylori was implicated as a carcinogen of the stomach by the World Health Organization in 1994. We had previously detected two gastric GIST cases in 2003; both of those had a positive finding of H pylori microorganisms and a positive CLO test for H pylori infections. However, this finding is more likely an incidental finding rather than a causal association.
GISTs are considered to be a group of mesenchymal neoplasms, and are also the subject of much debate and controversy regarding their nomenclature, histogenesis, criteria for diagnosis, prognostic manifestations, and classification[1,2]. Studies have revealed that some of these tumors may have tumor markers and features of neural, muscular or vascular endothelial differentiation. The term GIST has been adopted and defined as tumors arising from the stroma with no definite cell line of origin and varying patterns of differentiation[3]. Our GIST showed a mixed type of smooth muscle-neural origin. Some investigators emphasize the CD117 and CD34 expression in GISTs[1,4-6]. GISTs are now preferentially defined as tumors with c-kit (CD117) positive mesenchymal spindle cells or epithelioid neoplasms primarily in the gastrointestinal tract, omentum, and mesentery[4].
Statistically, GISTs are most common in the stomach (60-84.8%), followed by small intestine (10.5-30%), colon and rectum (3.5-5%), and esophagus (1.2-5%)[1,4]. The most important manifestation of stromal tumors is their indolent, slow growing nature. The tumors are generally found within the deeper stroma and the submucosa, and are often incidentally found during imaging studies. Our small GIST protruded about half of its mass into the upper posterior wall of the gastric fundus and only a 0.4 cm polypoid mass could be found in the endoscopic field.
GISTs often present with non-specific symptoms, such as nausea, vomiting, abdominal pain, gastrointestinal bleeding, and metastatic diseases. Bleeding is considered as the most common presentation of the clinical course[2]. Symptoms depend on tumor size and location. In our case, the GIST was totally asymptomatic. Ulcerations seem relevant to the size of GISTs. The size and mitotic score are considered as important diagnostic criteria and prognostic predictive indicators[1,2]. This GIST was diagnosed as a potential malignancy with a very low risk according to the pathological findings of mitosis (2 per 50 HPF) with combined smooth muscle-neural differentiation and positive stains for CD117 and CD34[1,2,5-7].
Generally, the preoperative rate of diagnosis of submucosal tumors by an endoscopic biopsy or an imaging study without skillful examination with an endoscopic ultrasonographic system and angiography is very low. Once a submucosal tumor is suspected, more careful endoscopic procedures, and repeat deep biopsy of the same area to reach the target tumor may have a higher diagnostic rate of histological findings, as our experience attests.
Patients with advanced GISTs which progress rapidly and result in organ destruction have poor prognosis. Therapeutic options for GISTs include surgery and treatment with STI-571 (Gleevec). STI-571 is a small molecule competitive inhibitor of the ATP binding site and demonstrates a high degree of specificity for inhibiting the activity of a small number of related tyrosine kinases: c-Ab1, Bcr-Ab1 (the molecular cause of chronic myeloid leukemia), platelet-derived growth factor receptors[8-10], and wild-type and mutant c-kit (stem cell factor receptor)[8,11,13]. This selective activity of STI-571 suggests that it has a relatively narrow target spectrum of anticancer activity. This highly selective molecule has a different therapeutic effect. Most malignant GISTs have mutant c-kit and some studies show that c-kit may be activated by mutation in three domains: extracellular, juxtamembrane and kinase portions (exon 9 or 11 or 13)[5,6,11,12]. STI-571 has the ability to inhibit signal transduction via c-kit and it is predictable that it should inhibit hematopoietic stem cells, resulting in neutropenia, anemia, thrombocytopenia, GI bleeding or intratumor hemorrhage[7].
Surgery is still the mainstay treatment for GISTs. Subtotal gastrectomy for the early gastric cancer with local resection for the small GIST was performed simultaneously for our patient with a small GIST concomitant with a IIc type of early gastric cancer.
In conclusion, we have reported a rare case of a small GIST combined with an early gastric cancer. It showed positive microscopic findings of H pylori microorganisms, a positive CLO test, and stainings for CD117 and CD34. More GIST cases are required for evaluating the relationship between H pylori infections and the etiologies of GIST concomitant with an early gastric cancer. Long-term observation for all GIST cases is greatly needed.
We greatly appreciate the financial and technical support of Dalin Tzu Chi General Hospital, Dr. Takahira Ikeda and the president of Japan Jichi Medical University, Professor Fumimaro Takaku who kindly refined the manuscript.
S- Editor Kumar M and Guo SY L- Editor Elsevier HK E- Editor Cao L
| 1. | Hasegawa T, Matsuno Y, Shimoda T, Hirohashi S. Gastrointestinal stromal tumor: consistent CD117 immunostaining for diagnosis, and prognostic classification based on tumor size and MIB-1 grade. Hum Pathol. 2002;33:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 157] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 2. | Yamada T. Textbook of Gastroenterology. 4th ed. Philadelphia: Lippincott Williams & Wilkins 2003; 1434. |
| 3. | Feldeman M, Scharschmidt BF, Sleisenger MH. Sleisenger & Fordtran’s Gastrointestinal and Liver Disease. 6th ed. Philadelphia: W.B. Saunders 1998; 751. |
| 4. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1173] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 5. | Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J. Gastrointestinal stromal tumors and leiomyosarcomas in the colon: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases. Am J Surg Pathol. 2000;24:1339-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 214] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000;15:1293-1301. [PubMed] |
| 7. | Duffaud F, Blay JY. Gastrointestinal stromal tumors: biology and treatment. Oncology. 2003;65:187-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000;96:925-932. [PubMed] |
| 9. | Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, Lydon NB. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000;295:139-145. [PubMed] |
| 10. | Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, Lydon NB. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2692] [Cited by in RCA: 2572] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 11. | Longley BJ, Reguera MJ, Ma Y. Classes of c-KIT activating mutations: proposed mechanisms of action and implications for disease classification and therapy. Leuk Res. 2001;25:571-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 220] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Lux ML, Rubin BP, Biase TL, Chen CJ, Maclure T, Demetri G, Xiao S, Singer S, Fletcher CD, Fletcher JA. KIT extracellular and kinase domain mutations in gastrointestinal stromal tumors. Am J Pathol. 2000;156:791-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 472] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 13. | Wang WL, Healy ME, Sattler M, Verma S, Lin J, Maulik G, Stiles CD, Griffin JD, Johnson BE, Salgia R. Growth inhibition and modulation of kinase pathways of small cell lung cancer cell lines by the novel tyrosine kinase inhibitor STI 571. Oncogene. 2000;19:3521-3528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 188] [Article Influence: 7.5] [Reference Citation Analysis (0)] |