Published online Feb 7, 2006. doi: 10.3748/wjg.v12.i5.796
Revised: August 9, 2005
Accepted: August 31, 2005
Published online: February 7, 2006
AIM: Interstitial cells of Cajal (ICCs) are the pacemaker cells that generate slow waves in the gastrointestinal (GI) tract. We have aimed to investigate the involvement of mitochondrial Na+-Ca2+ exchange in intestinal pacemaking activity in cultured interstitial cells of Cajal.
METHODS: Enzymatic digestions were used to dissociate ICCs from the small intestine of a mouse. The whole-cell patch-clamp configuration was used to record membrane currents (voltage clamp) and potentials (current clamp) from cultured ICCs.
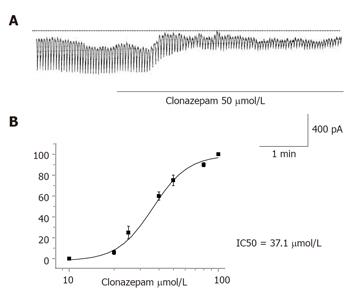
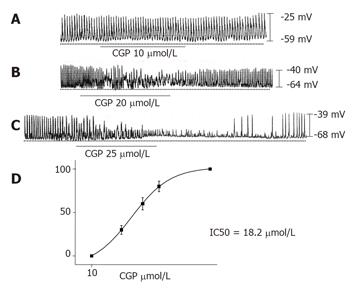
RESULTS: Clonazepam and CGP37157 inhibited the pacemaking activity of ICCs in a dose-dependent manner. Clonazepam from 20 to 60 µmol/L and CGP37157 from 10 to 30 µmol/L effectively inhibited Ca2+ efflux from mitochondria in pacemaking activity of ICCs. The IC50s of clonazepam and CGP37157 were 37.1 and 18.2 µmol/L, respectively. The addition of 20 µmol/L NiCl2 to the internal solution caused a “wax and wane” phenomenon of pacemaking activity of ICCs.
CONCLUSION: These results suggest that mitochondrial Na+-Ca2+ exchange has an important role in intestinal pacemaking activity.
- Citation: Kim BJ, Jun JY, So I, Kim KW. Involvement of mitochondrial Na+–Ca2+ exchange in intestinal pacemaking activity. World J Gastroenterol 2006; 12(5): 796-799
- URL: https://www.wjgnet.com/1007-9327/full/v12/i5/796.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i5.796
The interstitial cells of Cajal (ICCs) produce spontaneous rhythmic inward currents that are critical for the generation of slow waves in intestinal smooth muscle[1-3]. Pacemaker currents in ICCs result from the activation of a voltage-independent, non-selective cation conductance[4,5]. Pacemaking activity in ICCs is dependent upon metabolic activity[6] and Ca2+ release from intracellular stores[7]. Recent findings suggested that the pacemaker conductance in ICC is regulated by intracellular Ca2+ modulation[8]. The close association between IP3 receptor-dependent Ca2+ stores, mitochondria, and ion channels in the plasma membrane creates a basic cellular structure[9,10]. Release of Ca2+ from IP3 receptors does not directly initiate pacemaker currents in ICC, but rather, initiates Ca2+ uptake into the mitochondria. It was found that mitochondria in ICCs experience Ca2+ oscillations at the same frequency as pacemaker currents and that a rise in mitochondrial Ca2+ slightly precedes the activation of pacemaker currents[8]. This implies that pacemaker channels in the plasma membrane are activated by the falling phase of localized Ca2+ transients.
In isolated mitochondria, Ca2+ influx occurs via a Ca2+ uniporter driven by the membrane potential[11]. Ca2+ efflux occurs via Na+-Ca2+ exchange and can be inhibited by diltiazem, clonazepam, CGP37157, and by high external Ca2+[11-14]. However, the effect of inhibiting mitochondrial efflux, by using inhibitors of the Na+-Ca2+ exchange, on pacemaking activity of ICCs has not yet been investigated. Therefore, we undertook to investigate the involvement of mitochondrial Na+-Ca2+ exchange in pacemaking activity of ICCs.
Balb/c mice (8-13 days old) of either sex were anesthetized with ether and killed by cervical dislocation. The small intestines from 1 cm below the pyloric ring to the cecum were removed and opened along the mesenteric border. Luminal contents were removed by washing with Krebs-Ringer bicarbonate solution. The tissues were pinned to the base of a Sylgard dish and the mucosa removed by sharp dissection. Small tissue strips of the intestine muscle (consisting of both circular and longitudinal muscles) were equilibrated in Ca2+-free Hanks solution (containing in mmol/L: KCl 5.36, NaCl 125, NaOH 0.34, Na2HCO3 0.44, glucose 10, sucrose 2.9, and HEPES 11) for 30 min. Then, the cells were dispersed using an enzyme solution containing collagenase (Worthington Biochemical Co., Lakewood, NJ, USA) 1.3 mg/mL, bovine serum albumin (Sigma Chemical Co., St. Louis, MO, USA) 2 mg/mL, trypsin inhibitor (Sigma) 2 mg/mL and ATP 0.27 mg/mL. Cells were plated onto sterile glass coverslips coated with murine collagen (2.5 µg/mL, Falcon/BD, Franklin Lakes, NJ, USA) in a 35-mm culture dish and then cultured at 37 °C in a 95% O2, 50 mL/L CO2 incubator in a smooth muscle growth medium (Clonetics Corp., San Diego, CA, USA) supplemented with 2% antibiotics/antimycotics (Gibco, Grand Island, NY, USA) and murine stem cell factor (SCF, 5 ng/mL, Sigma). ICCs were identified immunologically with anti-c-kit antibody (phycoerythrin-conjugated rat anti-mouse c-kit monoclonal antibody; eBioscience, San Diego, CA, USA) at a dilution of 1:50 for 20 min[15]. ICCs were morphologically distinct from other cell types in the culture and thus it was possible to identify the cells by phase contrast microscopy once they had been verified with anti-c-kit antibody.
The whole-cell patch-clamp configuration was used to record membrane currents (voltage clamp) and potentials (current clamp) from cultured ICCs. An axopatch ID (Axon Instruments, Foster, CA, USA) was used to amplify membrane currents and potentials. The command pulse was applied using an IBM-compatible personal computer and pClamp software (version 6.1; Axon Instruments). Data obtained were filtered at 5 kHz and displayed on an oscilloscope, a computer monitor, and using a pen recorder (Gould 2200, Gould, Valley View, OH, USA).
Results were analyzed using pClamp and Origin (version 6.0) software. All experiments were performed at 30-32 °C.
The physiological salt solution used to bathe cells (Na+-Tyrode) contained (mmol/L): KCl 5, NaCl 135, CaCl2 2, glucose 10, MgCl2 1.2 and HEPES 10, adjusted to pH 7.4 with NaOH. The pipette solution contained (mmol/L): KCl 140, MgCl2 5, K2ATP 2.7, NaGTP 0.1, creatine phosphate disodium 2.5, HEPES 5 and EGTA 0.1, adjusted to pH 7.2 with KOH.
Before the development of CGP37157, several benzodiazepines (except clonazepam) were used as mitochondrial Na+-Ca2+ exchange inhibitors[12]. Clonazepam and CGP37157 were dissolved in dimethyl sulfoxide (DMSO) for 100 and 50 mmol/L stock solution, respectively and added (1 000 times dilution) to the bathing solution at the day of the experiment. The final concentration of DMSO in the bath solution was always <0.1%, and we confirmed that this concentration of DMSO did not affect the results that were recorded. Nickel chloride was directly added to the pipette solutions at the day of the experiment. CGP37157 was purchased from Tocris Cookson (Ellisville, MO, USA). The rest of the drugs were obtained from Sigma (Sigma Chemical Co., USA), unless otherwise stated. Diltiazem was not used because of its known effects on the cell membrane Ca2+ channels.
Effect of clonazepam on the pacemaking activity of ICCs Under a voltage clamp at a holding potential of -60 mV, clonazepam 50 µmol/L inhibited the pacemaking currents of ICCs (n = 4, Figure 1A). Clonazepam from 20 to 60 µmol/L effectively inhibited Ca2+ efflux from mitochondria on the pacemaking activity of ICCs. Concentrations of clonazepam of >100 µmol/L produced no further inhibition. The IC50 of clonazepam was 37.1 µmol/L (Figure 1B).
The benzodiazepine CGP37157 has been shown to be a more potent inhibitor than either clonazepam or diltiazem in terms of Ca2+ efflux measured in isolated mitochondria[12]. Thus, CGP37157 was applied to examine its effect on the pacemaking activity of ICCs. Under a current clamp (I = 0), CGP37157 was found to inhibit pacemaking potentials in a dose-dependent manner (n = 15, Figures 2A-2C). CGP37157 from 10 to 30 µmol/L effectively inhibited Ca2+ efflux from mitochondria on the pacemaking activity of ICCs. Concentrations of CGP37157 of >50 µmol/L produced no further inhibition. The IC50 of CGP37157 was 18.2 µmol/L (Figure 2D).
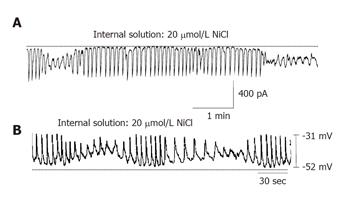
Ni2+ is a competitive inhibitor of the Ca2+ carrier, but it is not transported into the mitochondria[16]. Micromolar concentrations of nickel (Ni2+) chloride have been reported to inhibit Na+-Ca2+ exchange in both vascular and non-vascular cells[17]. In order to investigate the effect of NiCl2 on the pacemaking activity of ICCs, we added 20 µmol/L NiCl2 to the internal solution. Under a voltage clamp mode at a holding potential of -60 mV, the pacemaking activity of ICCs showed a “wax and wane” phenomenon (n = 6, Figure 3A). Also in current clamp mode (I = 0), the same phenomenon was shown (n = 3, Figure 3B). In case of 100 µmol/L NiCl2, the pacemaking activity of ICCs stopped (data not shown).
Intracellular Ca2+ plays an important role in the regulation of various cellular functions including exocytosis, metabolic activity, contractile activity, and gene expression in excitable cells. In these cells, the intracellular Ca2+ concentration ([Ca2+]i) is lowered by various mechanisms such as Ca2+ extrusion due to the actions of Na+-Ca2+ exchangers and Ca2+ pumps in the plasma membrane, Ca2+ uptake by Ca2+ pumps in the endoplasmic reticulum, and by Ca2+ uniporters in the mitochondria[17]. Sequestered Ca2+ in mitochondria is, in turn, released to the cytoplasma via various mechanisms[11]. Na+-Ca2+ exchangers and/or permeability transition pores are proposed to be involved in Ca2+ efflux from the mitochondria[11].
In case of ICCs, pacemaking activity is associated with mitochondrial Ca2+ transients. Pacemaker currents and rhythmic mitochondrial Ca2+ uptake by ICCs are blocked by inhibitors of IP3dependent Ca2+ release from the endoplasmic reticulum and by inhibitors of endoplasmic reticulum Ca2+ reuptake. Therefore, integrated Ca2+ management by endoplasmic reticulum and mitochondria is a prerequisite of electrical pacemaking in the gastrointestinal tract[8].
CGP37157 is a benzodiazepine derivative that inhibits electroneutral mitochondrial Na+-Ca2+ exchanger with submicromolar potency. In the heart, for example, this transporter is inhibited by CGP37157 at 400 nmol/L[12,14]. Before the development of CGP37157, several related benzodiazepines (e.g., clonazepam and diltiazem) were used as mitochondrial Na+-Ca2+ exchange inhibitors[14]. In general, few have reported that these compounds inhibit the cardiac plasmalemmal Na+-Ca2+ exchanger[18,19]. Mitochondrial Na+-Ca2+ exchanger protein participates in Ca2+ efflux and operates in opposition to a Ca2+ uniporter within the inner mitochondrial membrane. Thus, the inhibition of mitochondrial Na+-Ca2+ exchanger leads to an increase in Ca2+ levels within the mitochondria[14]. Calcium within the mitochondria serves as an important regulator of several key enzymes involved in energy metabolism. For example, the upregulation of the steady-state level of mitochondrial [Ca2+] ([Ca2+]m) result increases NADH production and stimulates oxidative phosphorylation[14].
The benzodiazepine CGP37157 has been shown to be a more potent inhibitor than either clonazepam or diltiazem on Ca2+ efflux, as measured in isolated mitochondria[12]. In ICCs, the IC50s of clonazepam and CGP37157 were found to be 37.1 and 18.2 µmol/L, respectively. Thus, CGP37157 was about twofold more potent than clonazepam.
Ni2+ is a potent inhibitor of mitochondrial Ca2+ transport[20] and a competitive inhibitor of Ca2+ carrier[16]. Also micromolar concentrations of nickel (Ni2+) chloride were found to inhibit Na+-Ca2+ exchanger in both vascular and non-vascular cells[21]. Therefore, we have investigated the effect of NiCl2 added to the internal solution. At 20 µmol/L, we observed a “wax and wane” phenomenon and at 100 µmol/L, the pacemaking activity of ICCs stopped.
Our data indicate that both clonazepam and CGP37157 inhibit the pacemaking activity of ICCs in a dose-dependent manner. The IC50s of clonazepam and CGP37157 were 37.1 and 18.2 µmol/L, respectively. When 20 µmol/L NiCl2 was added to the internal solution, the pacemaking activity of ICCs showed a “wax and wane” phenomenon.
We conclude that mitochondrial Na+-Ca2+ exchange has an important role in intestinal pacemaking activity.
S- Editor Guo SY L- Editor Elsevier HK E- Editor Li HY
| 1. | Langton P, Ward SM, Carl A, Norell MA, Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc Natl Acad Sci USA. 1989;86:7280-7284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 197] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91-97. [PubMed] |
| 3. | Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1032] [Cited by in RCA: 1072] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 4. | Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of cajal from the murine small intestine. J Physiol. 1998;513:203-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 223] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Thomsen L, Robinson TL, Lee JC, Farraway LA, Hughes MJ, Andrews DW, Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med. 1998;4:848-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 332] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | Nakayama S, Chihara S, Clark JF, Huang SM, Horiuchi T, Tomita T. Consequences of metabolic inhibition in smooth muscle isolated from guinea-pig stomach. J Physiol. 1997;505:229-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Liu LW, Thuneberg L, Huizinga JD. Cyclopiazonic acid, inhibiting the endoplasmic reticulum calcium pump, reduces the canine colonic pacemaker frequency. J Pharmacol Exp Ther. 1995;275:1058-1068. [PubMed] |
| 8. | Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000;525 Pt 2:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 250] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Sanders KM, Ördög T, Koh SD, Ward SM. A Novel Pacemaker Mechanism Drives Gastrointestinal Rhythmicity. News Physiol Sci. 2000;15:291-298. [PubMed] |
| 10. | Koh SD, Jun JY, Kim TW, Sanders KM. A Ca(2+)-inhibited non-selective cation conductance contributes to pacemaker currents in mouse interstitial cell of Cajal. J Physiol. 2002;540:803-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 123] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258:C755-C786. [PubMed] |
| 12. | Cox DA, Conforti L, Sperelakis N, Matlib MA. Selectivity of inhibition of Na(+)-Ca2+ exchange of heart mitochondria by benzothiazepine CGP-37157. J Cardiovasc Pharmacol. 1993;21:595-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 159] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Cox DA, Matlib MA. A role for the mitochondrial Na(+)-Ca2+ exchanger in the regulation of oxidative phosphorylation in isolated heart mitochondria. J Biol Chem. 1993;268:938-947. [PubMed] |
| 14. | Cox DA, Matlib MA. Modulation of intramitochondrial free Ca2+ concentration by antagonists of Na(+)-Ca2+ exchange. Trends Pharmacol Sci. 1993;14:408-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Goto K, Matsuoka S, Noma A. Two types of spontaneous depolarizations in the interstitial cells freshly prepared from the murine small intestine. J Physiol. 2004;559:411-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Bragadin M, Viola ER. Ni++ as a competitive inhibitor of calcium transport in mitochondria. J Inorg Biochem. 1997;66:227-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Bragadin M, Pozzan T, Azzone GF. Kinetics of Ca2+ carrier in rat liver mitochondria. Biochemistry. 1979;18:5972-5978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 113] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Takeo S, Elimban V, Dhalla NS. Modification of cardiac sarcolemmal Na+-Ca2+ exchange by diltiazem and verapamil. Can J Cardiol. 1985;1:131-138. [PubMed] |
| 19. | Hata T, Makino N, Nakanishi H, Yanaga T. Modulation of Na+-Ca2+ exchange in cardiac sarcolemmal vesicles by Ca2+ antagonists. Mol Cell Biochem. 1988;84:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Ligeti E, Bodnar J, Karoly E, Lindner E. Ni2+, a new inhibitor of mitochondrial calcium transport. Biochim Biophys Acta. 1981;656:177-182. [PubMed] |
| 21. | Tsang SY, Yao X, Wong CM, Au CL, Chen ZY, Huang Y. Contribution of Na+ -Ca2+ exchanger to pinacidil-induced relaxation in the rat mesenteric artery. Br J Pharmacol. 2003;138:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |