Published online Dec 28, 2006. doi: 10.3748/wjg.v12.i48.7874
Revised: April 28, 2006
Accepted: November 23, 2006
Published online: December 28, 2006
A 58-year-old female with a recurrent history of upper abdominal pain and intermittent dysphagia underwent endoscopic evaluation that demonstrated an irregular and nodular esophago-gastric (EG) junction and gradeIerosive esophagitis. Biopsies showed prominent intestinal metaplasia of Barrett’s type without dysplasia, chronic inflammation and multiple aggregates of large cells within the mucosal lamina propria, some with spindle shaped nuclei. Immunohistochemistry stains for keratins AE-1/AE-3 were negative, while S-100 and NSE were positive. This, together with routine stains, was diagnostic for mucosal ganglioneuromatosis. The background of chronic inflammation with intestinal type metaplasia was consistent with long-term reflux esophagitis. No evidence of achalasia was seen. Biopsies of gastric antrum and fundus were unremarkable, without ganglioneural proliferation. Colonoscopy was unremarkable. No genetic syndromes were identified in the patient including familial adenomatous polyposis and multiple endocrine neoplasia type IIb (MEN IIb). Iansoprazole (Prevacid) was started by oral administration each day with partial relief of symptoms. Subsequent esophagogastroscopy repeated at 4 mo showed normal appearing EG junction. Esophageal manometry revealed a mild non-specific lower esophageal motility disorder. Mild motor dysfunction is seen with gastro-esophageal reflux disease (GERD) and we feel that the demonstration of localized ganglioneuromatosis was not likely related etiologically. In the absence of findings that might suggest neural hypertrophy, such as achalasia, the nodular mucosal irregularity seen with this instance of ganglioneuromatosis may, however, have exacerbated the patient’s reflux.
- Citation: Siderits R, Hanna I, Baig Z, Godyn JJ. Sporadic ganglioneuromatosis of esophagogastric junction in a patient with gastro-esophageal reflux disorder and intestinal metaplasia. World J Gastroenterol 2006; 12(48): 7874-7877
- URL: https://www.wjgnet.com/1007-9327/full/v12/i48/7874.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i48.7874
This paper presents a solitary ganglioneuroma of the esophago-gastric junction in a 58-year old woman with a chief complaint of recurrent bouts of abdominal pain and mild dysphagia over several years and a recent onset of diarrhea. There are several primary esophageal tumors, which show neuroid differentiation, most arising from innate innervations of the esophagus.
These may be solitary or disseminated with Schwann cell and ganglion cell components involving any portion of the gastrointestinal tract. Cases may demonstrate either an exophytic polypoid or endophytic configuration and tend to arise from the neural plexus in the bowel wall. Non-neoplastic neural proliferations involving the esophagus include achalasia with inflammation and localized neural proliferation, which closely mimics ganglioneuromatosis. Neoplastic neural tumors that can involve the esophagus include ganglioneuroma, gastrointestinal autonomic nerve tumor (GAN), schwannoma, neurofibroma, granular cell tumor; and gangliocytic paraganglioma. Ganglioneuroma is a benign tumo, and the solitary variant may be cured by excision. Lesions in syndromic ganglioneuromatosis may require more extensive surgery.
The patient is a 58-year old Caucasian woman who presented with a complaint of recurrent bouts of abdominal pain, mild dysphagia over several years and recent onset of diarrhea. Previous medical history was significant for hyperlipidemia, insulin dependent diabetes mellitus, coronary artery disease, pancreatitis and diagnosed meningioma. Previous surgical history included cholecystectomy, appendectomy, cervical spine fusion and celiac axis stenting. Medicactions prior to endoscopic biopsy of esophagogastric EG junction included the following: Atorvastatin, pioglitazone HCl, diphenhydramine, Glipizide, Pyridoxine, isosobide and dihydrocodeinone.
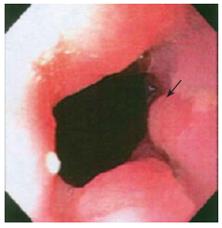
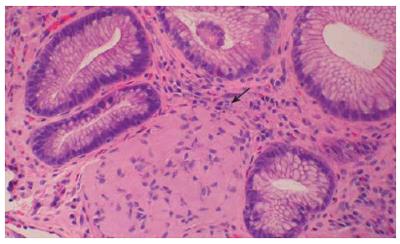
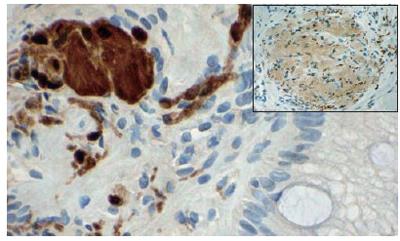
Endoscopy showed a somewhat nodular and irregular appearing EG junction with non-erosive mucosa (Figure 1). The antrum showed mild non-erosive gastritis. The pylorus and duodenum were unremarkable. Biopsies of the EG junction, antrum and duodenum were obtained. Hematoxylin and Eosin stained histologic sections from the EG junction showed aggregates of tangled fascicles of large cells mixed with Schwann like spindle cells expanding lamina propria (Figure 2). At low magnification this gave a pseudo granuloma appearance with distortion of the adjacent gland architecture. Higher magnification revealed a fibrillar cytoplasmic matrix with monomorphic bland nuclei without mitotic activity. Differential diagnosis for these features included ganglioneuroma, neurofibroma and schwannoma[1]. Immunohistochemical staining for S100 demonstrated a nerve sheath component. Focal positivity for Synaptophysin confirmed the presence of a ganglioneuronal tissue (Figure 3). The glandular epithelial component showed frequent goblet cells with no evidence of dysplasia. Chronic inflammatory cells were scattered in mucosal stroma. The biopsy diagnosis based on both HE and immunophenotypic characterization was of localized ganglioneuromatosis of EG junction within a background of mild chronic inflammation and prominent intestinal metaplasia without dysplasia.
Clinical correlation excluded the presence of achalasia or MEN-IIB. Treatment following this diagnosis of localized ganglioneuroma associated with esophagitis included prevacid 30 mg daily. Neurontin had been considered if symptoms would have progressed; however, this was not the case. A follow-up observation with repeat endoscopic evaluation at 4 mo was performed and showed mild gastritis with mild chronic inflammation in an unremarkable EG junction without ulceration or nodularity. Early recognition of symptomatology that might suggest either lower esophageal sphincter (LES) dysfunction or esophageal dysmotility was reviewed with the patient. No other family members were symptomatic or presented a history that might suggest hereditary or familial factors. Colonoscopy showed no discrete lesions.
Except for occasional bouts of mild non-specific abdominal pain and mild dysphagia, the patient remained well for a year. There was no complaint, odynophagia or nausea. Esophageal manometry was performed for persistent dysphagia at one year following the initial biopsy. This demonstrated a distal esophageal amplitude of 106 mm mercury with a duration of 3.5 s, peristaltic contraction 90% with 10% simultaneous contractions. Detailed review of the manometric data suggested a mild non-specific lower esophageal motility disorder.
In the absence of changes that might suggest neural hypertrophy, occasionally seen with achalasia, the mild motor dysfunction in this patient may be related to GERD. The demonstration of localized ganglioneuromatosis was most likely fortuitous and not etiologically related to the gastroesophageal reflux. However, the patient’s insulin dependant diabetes might have affected esophageal motility possibly associated with an increased incidence of reflux.
It is most likely that the generous biopsy sampling of the nodular EG may have removed a significant portion of the lesion; therefore, it was not overtly visible on the subsequent endoscopy.
Primary esophageal tumors that show neuroid differen-tiation in addition to ganglioneuromata, include gastro-intestinal autonomic nerve tumor (GAN)[2,3], schwannoma[4,5] (some occasionally showing melanocytic differentiation)[6], and neurofibromas[7]. The innervation of the lower esophagus includes parasympathetic supply from the vagi and sympathetic innervation from the greater splanchnic and thoraci ganglia[8]. Histologically the neural components are seen within the muscle layer as myenteric plexus and in the submucosal neural plexus with branches entering the lamina propria[9].
Solitary or disseminated Schwann cell and ganglion cell proliferation anywhere in the gastrointestinal tract may appear as small intramucosal nodular lesions[10], exophytic polypoid lesions, or poorly demarcated transmural proliferations[11]. Ganglioneuroma, a fully differentiated tumor with no immature components[12], may occur as a solitary lesion (sporadic) or as multiple lesions called ganglioneuromatosis and may be associated with other diseases (syndromic).
Sporadic ganglioneuroma has been unknown to be associated with genetic syndromes and has been detected in patients of all ages with a mean age of 50 years. The majority of the solitary lesions are asymptomatic and, therefore, found incidentally, most frequently in the left colon[13].
Among the cases of multiple lesions, ganglione-uromatosis of an exophytic polypoid type (ganglione-uromatous polyposis) is characterized by interposition of neural proliferations with glandular components and is usually associated with familial adenomatous polyposis and multiple cutaneous lipomas. Ganglioneuromatosis of transmural proliferation type arising from the neural plexus in the bowel wall is frequently associated with other tumors, including MEN IIb (medullary carcinoma thyroid, pheochromocytoma, oral-mucosal neuromas and skeletal deformities)[14], multiple ganglioneuromas and neurofibromas of the gastrointestinal tract, von Recklinghausen’s disease, and neurogenic sarcoma[15]. Florid hyperplasia of submucosal or myenteric plexus is distinct for intramural ganglioneuromatosis and occurs with typeIneurofibromatosis[16]. Patients with syndromic ganglioneuromatosis present with symptoms, and the lesions are found much earlier in life, with a mean age approximately 35 years. There is no gender predominance in the incidence of this disease.
Non neoplastic neural proliferations involving the esophagus include achalasia, which is an esophageal motor disorder associated with a loss of myenteric ganglion cells with inflammation and secondary changes including neural proliferation, which closely mimics ganglioneuromatosis[17]. Neoplastic neural tumors that can involve the esophagus include ganglioneuroma, GAN, schwannoma, neuro-fibroma and other less prevalent forms like granular cell tumor with large eosinophilic cells[18]; gangliocytic paraganglioma showing predominantly spindle shaped cells with both ganglion and neuroendocrine features (more often seen in duodenum)[19]. Ganglioneuromatosis is visibly present throughout the gut showing predominantly spindle shaped neural proliferations with frequent ganglion type cells.
Ganglioneuroma is a benign tumor and the solitary variant may be cured by excision of the nodular lesion or complete polypectomy. Lesions in syndromic ganglioneuromatosis may require surgery, but the patient may die from the associated syndromes.
In summary, this case illustrates an instance of sporadic ganglioneuromatosis involving EG junction in a 58-year old female with a recurrent history of upper abdominal pain. Background of chronic inflammation and intestinal type metaplasia suggested that the symptoms were related to reflux esophagitis, possibly exacerbated by the nodular growth of the ganglioneuromatosis, which involved the gastro-esophageal junction. The attendant mild lower esophageal motility disorder (demonstrated by manometry) may reflect nonspecific reflux disorder. The patient was initially treated with Iansoprazole (Prevacid), which resulted in a partial relief of symptoms. This was followed by esomeprazole (Nexium) with additional relief of symptoms. The patient continued to experience mild intermittent dysphagia without odynophagia or nausea. Repeat EG endoscopy at 4 mo showed unremarkable GE junction. The importance of recognizing symptomatology indicative of lower esophageal sphincter dysfunction was reviewed with this patient with a discussion of follow-up studies over time[20]. It appears that the initial generous biopsy sampling removed the EG tumor nodularity, and this, together with the anti-acid treatment, decreased the intensity of the reflux esophagitis. Following the manometric study the patient was advised to swallow slowly with at least 30 s intervals, avoid beverages with extreme temperatures and was maintained on her current medications. We believe that although not etiologically associated, localized esophageal ganglioneuromatosis may exacerbate aspects of gastro-esophageal reflux disorder.
S- Editor Wang GP L- Editor Zhu LH E- Editor Liu WF
| 1. | Burger PC, Scheithaur BW, Vogel FS. Surgical Pathology of the Nervous System and its Coverings. 4th ed. New York: Churchill Livingston 2002; 515-519, 620-623. |
| 2. | Lam KY, Law SY, Chu KM, Ma LT. Gastrointestinal autonomic nerve tumor of the esophagus. A clinicopathologic, immunohistochemical, ultrastructural study of a case and review of the literature. Cancer. 1996;78:1651-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol. 1999;30:1213-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 522] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 4. | Hsu SD, Cheng YL, Chen A, Lee SC. Esophageal schwannoma. J Formos Med Assoc. 2003;102:346-348. [PubMed] |
| 5. | Prévot S, Bienvenu L, Vaillant JC, de Saint-Maur PP. Benign schwannoma of the digestive tract: a clinicopathologic and immunohistochemical study of five cases, including a case of esophageal tumor. Am J Surg Pathol. 1999;23:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 98] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Brown RM, Darnton SJ, Papadaki L, Antonakopoulos GN, Newman J. A primary tumour of the oesophagus with both melanocytic and schwannian differentiation. Melanocytic schwannoma or malignant melanoma. J Clin Pathol. 2002;55:318-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Lee R, Williamson WA. Neurofibroma of the esophagus. Ann Thorac Surg. 1997;64:1173-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Young B, Heath JW. Gastrointestinal tract structure. 4th ed. Philadelphia: Churchill Livingston 2000; 250-251. |
| 11. | Kwon MS, Lee SS, Ahn GH. Schwannomas of the gastrointestinal tract: clinicopathological features of 12 cases including a case of esophageal tumor compared with those of gastrointestinal stromal tumors and leiomyomas of the gastrointestinal tract. Pathol Res Pract. 2002;198:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Weis WW, Goldblum JR. Primaive Neuroecodermal Tumors and Related Lesions. 4th ed. Philadelphia: Mosby 2001; 1284-1285. |
| 13. | Iacobuzio-Donahue CA, Montgomery E, Goldblum JR. Gastrointestinal Mesenchymal Tumors Ch. 7, edited by Montgomery E, Fisher C. Philadelphia: Churchill Livingstaon 2005; 204-234. |
| 14. | Cuthbert JA, Gallagher ND, Turtle JR. Colonic and oesophageal disturbance in a patient with multiple endocrine neoplasia, type 2b. Aust N Z J Med. 1978;8:518-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Lewin KJ, Appelman HD. Mesenchymal tumors and tumor-like proliferations. Washington DC: AFIP Press 1996; 441-444. |
| 16. | Weidner N, Cote R, Suster S, Weiss L. Gastrointestinal tract; Large Intestine. Philadelphia: Saunders 2003; 792-795. |
| 17. | Mills SE, Carter D, Greenson JK, Oberman HA, Reuter V, Stoler MH. Inestinal Neoplasms Ch. 34. Vol. 2, 4th edition. Philadelphia: Lipnncott Williams&Wilkins 2004; 1549-1550, (achalasia) 1413. |
| 18. | Gershwind ME, Chiat H, Addei KA, Ferraro LR. Granular cell tumors of the esophagus. Gastrointest Radiol. 1978;2:327-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Odze R, Goldblum J, Crawford J. Mesenchymal Tumors of the GI tract Ch. 22. Philadelphia: Saunders 2004; 518-519. |
| 20. | Fauci AS, Braunwald E, Isselbacher K, Wilson JD, Martin JB, Kasper DL, Hauser SL, Longo DL. Diseases of the Esophagus Ch. 283. 14th edition. New York: MxGraw-Hill 1998; 1588-1596. |