Published online Dec 28, 2006. doi: 10.3748/wjg.v12.i48.7737
Revised: August 28, 2006
Accepted: September 19, 2006
Published online: December 28, 2006
Patients with liver disease may present hepatic enceph-alopathy (HE), a complex neuropsychiatric syndrome covering a wide range of neurological alterations, including cognitive and motor disturbances. HE reduces the quality of life of the patients and is associated with poor prognosis. In the worse cases HE may lead to coma or death.
The mechanisms leading to HE which are not well known are being studied using animal models. The neurological alterations in HE are a consequence of impaired cerebral function mainly due to alterations in neurotransmission. We review here some studies indicating that alterations in neurotransmission associated to different types of glutamate receptors are responsible for some of the cognitive and motor alterations present in HE.
These studies show that the function of the signal transduction pathway glutamate-nitric oxide-cGMP associated to the NMDA type of glutamate receptors is impaired in brain in vivo in HE animal models as well as in brain of patients died of HE. Activation of NMDA receptors in brain activates this pathway and increases cGMP. In animal models of HE this increase in cGMP induced by activation of NMDA receptors is reduced, which is responsible for the impairment in learning ability in these animal models. Increasing cGMP by pharmacological means restores learning ability in rats with HE and may be a new therapeutic approach to improve cognitive function in patients with HE. However, it is necessary to previously assess the possible secondary effects.
Patients with HE may present psychomotor slowing, hypokinesia and bradykinesia. Animal models of HE also show hypolocomotion. It has been shown in rats with HE that hypolocomotion is due to excessive activation of metabotropic glutamate receptors (mGluRs) in substantia nigra pars reticulata. Blocking mGluR1 in this brain area normalizes motor activity in the rats, suggesting that a similar treatment for patients with HE could be useful to treat psychomotor slowing and hypokinesia. However, the possible secondary effects of mGluR1 antagonists should be previously evaluated.
These studies are setting the basis for designing therapeutic procedures to specifically treat the individual neurological alterations in patients with HE.
- Citation: Felipo V. Contribution of altered signal transduction associated to glutamate receptors in brain to the neurological alterations of hepatic encephalopathy. World J Gastroenterol 2006; 12(48): 7737-7743
- URL: https://www.wjgnet.com/1007-9327/full/v12/i48/7737.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i48.7737
Hepatic encephalopathy (HE) is a complex neuropsy-chiatric syndrome present in patients with chronic or acute liver disease. HE covers a wide range of neuropsychiatric disturbances ranging from minimal changes in personality or altered circadian rhythms (sleep-waking cycle) to alterations in intellectual function, personality, conscience and neuromuscular coordination. HE is usually reversible, but can lead to coma and death in the worse case.
The neurological alterations in HE are the result of a previous liver failure. Liver failure leads to impaired detoxification of ammonia and other toxic substances that can reach the brain and alter its function. Many studies have been carried out to identify factors responsible for the neurological alterations in HE. Clinical experience and basic research indicate that ammonia is the main factor responsible for HE. Ammonia is a product of degradation of proteins and other nitrogenated compounds but at high concentrations ammonia is toxic, leading to alteration of cerebral function which can lead to coma and death.
Hyperammonemia is therefore considered the main factor contributing to the neurological alterations found in HE both in acute and in chronic liver disease[1]. Other factors may also contribute to neurological alterations in HE. It has been recently suggested that inflammation exacerbates the neuropsychological effects induced by hyperammonemia in cirrhosis[2].
Classical clinical treatment of HE is mainly directed to reducing ammonia concentration by reducing protein ingestion, lowering ammonia production by the intestinal bacteria as well as by reducing ammonia transport from intestine to the blood flow by acidification of the intestinal lumen.
Overt HE is usually elicited by one of the following precipitating factors (high protein ingestion, gastrointesti-nal constipation, bleeding, diuretics) usually associated with increased ammonia levels. HE is associated with a poor prognosis[3,4].
Liver cirrhosis patients with normal neurological and mental status may present minimal forms of HE, showing intellectual function impairment which cannot be detected through general clinical examination but can be unveiled using specific neuropsychological or neurophysiological examination[5,6]. Minimal hepatic encephalopathy (MHE) is the first stage in the spectrum of HE[7,8]. Patients suffering from MHE may present psychomotor slowing and cognitive deficits affecting their ability to perform certain activities in daily life. MHE is therefore associated with impaired quality-of-life[9,10]. Patients with MHE and altered oral glutamine challenge have a shortened life-span[7].
Treatment of MHE could prevent or delay the appearance of clinical HE and improve quality of life and life span of patients. In order to detect the presence of MHE and to treat it efficiently, it is necessary to know the mechanisms leading to MHE and subsequently to HE.
The mechanisms leading to HE are mainly studied using animal models of chronic liver failure and chronic hyperammonemia. The neurological alterations in HE are a consequence of impaired cerebral function mainly due to alterations in neurotransmission. Alterations in different neurotransmitter systems (glutamatergic, GABAergic, serotoninergic, etc) have been reported in HE[1].
We review here some studies indicating that alterations in neurotransmission associated to different types of glutamate receptors are responsible for some of the cognitive and motor alterations present in HE. Pharmacological manipulation of these receptors or pathways may normalize cognitive and motor function in animal models of HE.
Cognitive, motor and sleep alterations (impairment of sleep-wake cycle) are commonly observed in patients with HE and their intensities vary with the grade of HE.
Alterations in the regulation of biological rhythms such as sleep, appetite, melatonin production and in sexuality are common in patients with liver disease[11-14].
Patients with HE show alterations in cognition, consciousness, attention, memory, and learning. Cirrhotic patients with minimal HE are “clinically normal” but present cognitive alterations which can be unveiled by a detailed analysis of the patients’ history and by neurophysiological and neuropsychological assessment of consciousness and sensory, cognitive and motor functions[5,6]. The prevalence of minimal HE in patients with liver disease ranges from 30% to 84% depending on the kind and number of tests used and the population (etiology and severity of the liver disease) investigated. Even minimal HE is associated with reduced quality of life and ability to work and drive[9,15-17]. Moreover, patients suffering minimal HE have increased probability of suffering later overt HE[7,18,19].
Patients with minimal HE show impaired ability to perform memory tasks, mainly because of deficits in attention and visual perception[20,21]. These patients also perform worse than healthy controls in motor function, visual perception, visual orientation and visual-constructive abilities[22-25].
Sustained attention is also impaired in cirrhotic patients even when memory, language, or motor alterations are absent[21,26,27]. Patients with minimal HE have the tendency to be easily distracted.
Early manifestations of intellectual dysfunction in HE patients include psychomotor slowing and impaired ability to perform tasks that require sustained attention[18,26,28]. As encephalopathy worsens, impairment in speech and inability to copy simple drawings (e.g. a star) appear. Patients in gradeIIHE show temporal and spatial disorientation and reduced vigilance state or delirium. Grade IV HE is characterized by the appearance of stupor and coma.
Patients with HE show also altered motor function and coordination, including psychomotor slowing and hypokinesia which was attributed to alterations in basal ganglia[29]. Motor alterations include increased muscular tone, reduced speed of rapid alternating movement, ataxia, an increased deep tendon reflexes, abnormal movements such as tremors, particularly asterixis. Also hypomimia, dysarthria, bradykinesia and hypokinesia could be detected on careful neurological examination.
The mechanisms by which liver failure leads to altered intellectual and motor function remain unclear. Identifying these mechanisms would allow designing treatments to improve intellectual and motor function in patients with HE.
Some motor and cognitive alterations present in HE patients are reproduced in animal models of chronic liver failure (e.g. rats with portacaval anastomosis) and chronic hyperammonemia[30-38]. These animal models are being used to study the molecular mechanisms by which liver disease leads to HE and altered intellectual and motor function. Once these mechanisms are identified, new studies begin to assess whether similar alterations and mechanisms occur in brain of patients with HE.
Glutamate is the main excitatory neurotransmitter in mammals and modulates important cerebral processes including cognitive and motor functions (see below). Glutamatergic neurotransmission involves several steps, beginning with release of glutamate from the presynaptic neuron. Glutamate in the extracellular space activates glutamate receptors present in the synaptic membranes, leading to activation of signal transduction pathways associated to these receptors. To avoid continuous activation of glutamate receptors, glutamate is removed from the synaptic cleft by specific glutamate transporters located mainly in astrocytes. All these steps are tightly modulated under physiological conditions and alterations of any of the above steps may result in impairment of glutamatergic neurotransmission, leading to neurological alterations. Some of the parameters that can be altered under pathological conditions are: (1) the content (expression, synthesis and/or degradation) of the main proteins involved in glutamatergic neurotransmission (e.g. different types of glutamate receptors or transporters); (2) the regulation of the spatial location of the receptors and transporters. Only the receptors or transporters present in the membrane can recognize extracellular glutamate. Many of these proteins are associated to formation of clusters to improve the yield of the neurotransmission process; (3) the function of the receptors and transporters, which is modulated in different ways including phosphorylation-dephosphorylation, binding of co-agonists, etc; (4) alterations in the release or uptake of glutamate may result in altered extracellular glutamate, leading to altered neurotransmission; (5) alterations in any of the steps of the signal transduction pathways associated with the different types of glutamate receptors would also result in impaired glutamatergic neurotransmission.
There is therefore a large number of possible sites or molecular targets for interference by hyperammonemia or liver disease of glutamatergic neurotransmission. Hyperammonemia and liver failure alter different steps of glutamatergic neurotransmission including: glutamate concentration in the extracellular fluid in brain, transport and transporters of glutamate, content and function of different types of glutamate receptors and signal transduction pathways associated to these receptors[39].
Glutamatergic neurotransmission plays an important role in modulating intellectual function (learning and memory), motor function and coordination and circadian rhythms. As mentioned above, these processes are altered in patients with liver disease and HE, who show altered sleep-waking patterns, motor function and coordination and decreased intellectual capacity. The alterations in glutamatergic neurotransmission may be responsible for some of these neurological alterations found in HE patients.
We summarize below the alterations in glutamatergic neurotransmission that have been shown to contribute to the cognitive and motor alterations in hepatic encephalopathy.
Glutamate has two main types of receptors: ionotropic and metabotropic. Activation of ionotropic glutamate receptors leads to the opening of ion channels allowing the transport of Na+, K+ through them, and Ca2+ in some cases. There are three main types of ionotropic glutamate receptors: NMDA, AMPA and kainate receptors. The NMDA type of glutamate receptors is involved in the control of cerebral processes such as neuronal plasticity, learning and memory. Alterations in signal transduction pathways associated to NMDA receptors are involved in the impairment in cognitive function in HE (see below).
Metabotropic glutamate receptors (mGluRs) are coupled to G proteins. Activation of mGluRs modulates the activity of different enzymes (phospholipase C, adenylate cyclase, etc) and ion channels through these G proteins, resulting in modulation of the intracellular levels of second messengers such as diacylglycerol, inositol triphosphates, cAMP, etc. These second messengers in turn, modulate the activity of other enzymes (protein kinases C and A, etc) that continue the transmission of the signal induced by activation of metabotropic glutamate receptors.
Metabotropic glutamate receptors are involved in modulation of motor function. Alterations in activation of metabotropic glutamate receptors are involved in some motor alterations in HE (see below).
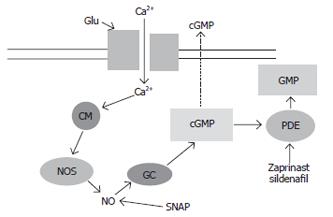
Activation of NMDA receptors by glutamate leads to increased intracellular Ca2+ in the post-synaptic neurons. Ca2+ binds to calmodulin (CM) and activates different enzymes, including neuronal nitric oxide synthase (NOS), leading to increased formation of nitric oxide (NO), which in turn activates soluble guanylate cyclase (GC) and increases cGMP (Figure 1). Part of the cGMP formed is released to the extracellular space. Under appropriate conditions the increase in extracellular cGMP is a good measure of the extent of activation of NMDA receptors in brain in vivo.
The function of this glutamate-nitric oxide-cGMP pathway is impaired in brain in vivo in animal models of HE (rats with chronic hyperammonemia or with chronic liver failure). The most usual animal model for studying the neurological alterations in HE is the rat with chronic liver failure induced surgically by portacaval anastomosis. This model reproduces some of the neurological alterations found in patients with HE. Liver failure induces, in addition to hyperammonemia, other alterations (decreased muscle mass, altered metabolism of other compounds, etc). To discern the contribution of hyperammonemia to the neurological alterations in HE, we developed an animal model of hyperammonemia without liver failure: rats fed an ammonium-containing diet[40,41]. These rats present a level of hyperammonemia similar to that of patients with liver cirrhosis or of rats with portacaval anastomosis, but do not present other alterations associated to liver failure and may be considered therefore as a model of “pure” hyperammonemia. Comparison of the effects induced by both models can clarify which effects are due to hyperammonemia and which are due to other factors associated to liver failure.
Using this model we found that chronic moderate hyperammonemia without liver failure impairs the function of the glutamate-NO-cGMP pathway in cerebellum in vivo, as shown by brain microdialysis in freely moving rats[42]. When microdialysis probes are inserted in the cerebellum of control or hyperammonemic rats without liver failure, administration of NMDA through the microdialysis probe activates the glutamate-NO-cGMP pathway and increases cGMP formation (Figure 1). The NMDA-induced increase in extracellular cGMP in cerebellum is significantly lower in hyperammonemic rats than in control rats[42], indicating that chronic hyperammonemia impairs the function of the glutamate-NO-cGMP pathway in rat cerebellum in vivo.
To assess whether the impairment occurs at the level of activation of soluble guanylate cyclase by NO, a NO-generating agent, SNAP (Figure 1) was administered through the microdialysis probe to directly activate guanylate cyclase. The increase in extracellular cGMP induced by SNAP was also significantly reduced in hyperammonemic rats. This indicates that chronic moderate hyperammonemia impairs activation of soluble guanylate cyclase by NO and function of the glutamate-NO-cGMP pathway in cerebellum in vivo.
Chronic liver failure induced by portacaval anastomosis, also impairs the glutamate-NO-cGMP pathway in cerebellum in vivo, as shown by brain microdialysis in freely moving rats by Monfort et al[43]. NMDA-induced increase in extracellular cGMP in cerebellum was significantly lower in rats with portacaval anastomosis than in control rats. These results indicate that the function of the glutamate-NO-cGMP pathway is impaired in cerebellum in vivo in animal models of HE. Moreover, one of the steps of the pathway affected is the activation of soluble guanylate cyclase by NO.
To assess whether activation of soluble guanylate cyclase by NO is also altered in cerebellum of cirrhotic patients with HE, we measured the activation of soluble guanylate cyclase by the NO-generating agent SNAP in homogenates of cerebellum from controls and cirrhotic patients who died of hepatic coma. The activation of guanylate cyclase by the NO-generating agent SNAP was significantly lower in cerebellum from cirrhotic patients than in cerebellum from controls[44].
The above results show that animal models of HE reproduce faithfully the alterations in the modulation of guanylate cyclase by NO present in cerebellum of patients died of HE, which supports the idea that hyperammonemia is responsible for these alterations.
Cognitive function and learning ability are impaired in patients with liver disease (see above) and in animal models of HE[35-37]. The glutamate-nitric oxide-cGMP pathway and cGMP modulate some forms of learning and memory. It is therefore likely that the impairment in the function of the glutamate-nitric oxide-cGMP pathway in brain may contribute to the cognitive impairment in HE patients.
We hypothesize that the alterations in the function of the glutamate-nitric oxide-cGMP pathway and the decrease in extracellular cGMP in brain may be responsible for the impairment in learning ability and intellectual function in HE patients, and that pharmacological modulation of extracellular cGMP concentration may restore learning ability in patients with hyperammonemia and HE.
To assess this possibility we tested whether pharmac-ological treatments directed to increase extracellular cGMP in brain are able to reverse the impairment in learning ability in rat models of HE. We were able to increase extracellular cGMP and completely restore learning ability of the rats by using three different treatments: continuous intracerebral administration of zaprinast, an inhibitor of the phosphodiesterase that degrades cGMP[36]; chronic oral administration of sildenafil, an inhibitor of the phosphodiesterase that crosses the blood-brain barrier[35], and continuous intracerebral administration of cGMP[36].
The above data indicate that the impairment of learning ability (at least of the ability to learn the Y maze conditional discrimination task) in animal models of HE is due to impairment of the glutamate-nitric oxide-cGMP pathway. As the function of this pathway is also altered in brain of patients with liver cirrhosis, this alteration should also contribute to the cognitive impairment in these patients.
Increasing extracellular cGMP by pharmacological means may be a new therapeutic approach to improve learning and memory performance both in patients with evident HE and cognitive impairment and in patients with minimal HE who present reduced performance in psychometric tests.
The neurological alterations in patients with HE also include alterations in motor function and coordination. One of the early alterations in patients with liver disease is psychomotor slowing. Jover et al[29] reported that 41% of patients with liver cirrhosis show hypokinesia, which is attributed to alterations in basal ganglia. These patients may also present bradykinesia. Rats with chronic liver failure due to portacaval anastomosis also show reduced motor activity which is similar to the motor slowing, hypokinesia and bradykinesia present in patients with HE[38,45-48]. We used this animal model to study the mechanisms involved in motor alterations in HE patients.
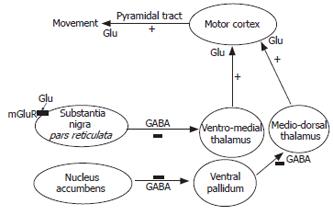
The neuronal circuits between basal ganglia and prefrontal cortex are essential in the modulation of motor function. This network includes basal ganglia, motor thalamus and cerebral cortex (Figure 2). The basal ganglia (including nucleus accumbens) produces signals that go to the thalamus which sends signals to the cortex to modulate movement execution. The signals originated in the thalamus are modulated by substantia nigra pars reticulata (SNr), which sends inhibitory projections to the ventromedial thalamus (VMT).
Patients with liver disease show hyperintensities in magnetic resonance imaging in basal ganglia nuclei[49-51], suggesting altered function of these nuclei, which is also supported by PET studies[52]. It has been suggested that the motor symptoms of HE are a consequence of basal ganglia dysfunction and alterations affecting the function of the neuronal circuits between basal ganglia and cerebral cortex[5,49-53].
The motor activity mediated by the basal ganglia-thalamus-cortex circuit is modulated by glutamatergic neurotransmission in nucleus accumbens and SNr (Figure 2). Activating metabotropic glutamate receptors in nucleus accumbens induces motor activity. Activation of glutamate receptors in SNr induces hypolocomotion in normal rats[54-56], while glutamate receptor antagonists administered in SNr induce hyperlocomotion[56,57]. Alterations in glutamatergic neurotransmission in SNr therefore may contribute to the psychomotor slowing and hypokinesia in HE patients.
We analysed the neurochemical alterations in the basal ganglia-thalamus-cortex circuit by in vivo brain microdialysis in rats with portacaval anastomosis and correlated the alterations in neurotransmitters (glutamate, GABA) with the alterations in motor function. Moreover we tried to normalize motor function of rats with portacaval anastomosis by pharmacologically modulating glutamatergic neurotransmission.
Extracellular glutamate is significantly increased (15-fold) in SNr of rats with portacaval shunt (PCS) and its motor activity is reduced by 40%. There is a significant negative correlation between locomotor activity and extracellular glutamate in SNr, indicating that increased glutamate is responsible for hypolocomotion in PCS rats[38].
We hypothesize that hypolocomotion in PCS rats is due to over-activation of metabotropic glutamate receptors (mGluRs) by the increased extracellular glutamate. To assess this possibility, we tested whether blocking mGluR1 with the antagonist CPCCOEt can normalize the motor activity in PCS rats and found that blocking mGluR1 does not affect the motor activity in control rats but increases it in PCS rats to reach the same motor activity than control rats[38]. The above data show that increased activation of the metabotropic glutamate receptor mGluR1 in SNr is responsible for hypolocomotion in rats with chronic liver failure. We studied the possible mechanisms involved in this increased activation. The increased activation of mGluR1 is not due to increased amount of the receptor, which is significantly reduced in SNr of PCS rats[38], indicating that enhanced activation of the metabotropic receptor is due to the increase in the extracellular concentration of glutamate.
The increase in extracellular glutamate could be due to increased release, reduced uptake or both. We analysed the content of the two main transporters that take up glutamate: EAAC-1 and GLT-1. The content of both transporters is reduced by 23%-27%[38]. The reduced content of glutamate transporters may contribute to increased extracellular glutamate in SNr. Enhanced glutamate release could also contribute to the increased extracellular glutamate.
The increased activation of metabotropic glutamate receptors in SNr results in altered function of the whole basal ganglia-thalamus-cerebral cortex circuit (Figure 2), and therefore in hypolocomotion. Increased mGluR1 activation in SNr leads to increased GABA concentration in ventro-medial thalamus, which mediates hypolocomotion in PCS rats. Blocking mGluR1 in SNr normalizes GABA in ventro-medial thalamus and locomotion. The circuit by which SNr modulates motor activity also involves glutamate in motor cortex. Blockade of mGluR1 in SNr increases glutamate in motor cortex and activity.
There are reports supporting that changes similar to those summarized above in rats with chronic liver failure also may occur in patients with chronic liver disease. Alterations in the function of basal ganglia in liver cirrhosis are supported by the hyperintensities in magnetic resonance images found consistently in these patients[49-51]. Altered function of the thalamus is also supported by PET studies in patients with HE[52]. It is therefore likely that excessive activation of metabotropic glutamate receptors in SNr may be also involved in the psychomotor slowing and hypokinesia in patients with HE.
These studies may have clinical implications. Blocking mGluR1 in SNr normalizes motor activity in a rat model of HE, suggesting that a similar treatment for patients with HE can be used to treat psychomotor slowing and hypokinesia in these patients. However, the possible effects of mGluR1 antagonists on other cerebral functions should be previously evaluated. Each type of glutamate receptors and their associated signal transduction pathways may be expressed in different brain areas where they may modulate different cerebral functions. This implies that, for example, blocking mGluR1 in SNr may restore motor function in patients with liver disease, but blocking it in another brain area may impair some other cerebral functions. Before trying to apply these treatments in humans, careful studies in animal models are therefore required to analyse the possible secondary effects, and ideally, if possible, to develop appropriate delivery procedures allowing to modulate specific receptors or pathways in specific brain areas without affecting its function in other areas.
In summary, the studies reviewed here show that alterations in signal transduction pathways associated to the NMDA type of ionotropic glutamate receptors are involved in cognitive impairment in HE while alterations in activation of metabotropic glutamate receptors are involved in the motor alterations. Moreover, pharmacological manipulation of the altered pathways and receptors can normalize both cognitive and motor functions in animal models of HE. These studies are setting the basis for designing therapeutic procedures to specifically treat individual neurological alterations in patients with HE.
S- Editor Wang J L- Editor Wang XL E- Editor Bai SH
| 1. | Felipo V, Butterworth RF. Neurobiology of ammonia. Prog Neurobiol. 2002;67:259-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 454] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 2. | Shawcross DL, Davies NA, Williams R, Jalan R. Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J Hepatol. 2004;40:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 381] [Article Influence: 18.1] [Reference Citation Analysis (1)] |
| 3. | Bustamante J, Rimola A, Ventura PJ, Navasa M, Cirera I, Reggiardo V, Rodés J. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30:890-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 368] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 4. | Hui AY, Chan HL, Leung NW, Hung LC, Chan FK, Sung JJ. Survival and prognostic indicators in patients with hepatitis B virus-related cirrhosis after onset of hepatic decompensation. J Clin Gastroenterol. 2002;34:569-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Amodio P, Montagnese S, Gatta A, Morgan MY. Characteristics of minimal hepatic encephalopathy. Metab Brain Dis. 2004;19:253-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 203] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1410] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 7. | Romero-Gómez M, Boza F, García-Valdecasas MS, García E, Aguilar-Reina J. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol. 2001;96:2718-2723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 261] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 8. | Das A, Dhiman RK, Saraswat VA, Verma M, Naik SR. Prevalence and natural history of subclinical hepatic encephalopathy in cirrhosis. J Gastroenterol Hepatol. 2001;16:531-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 203] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Groeneweg M, Quero JC, De Bruijn I, Hartmann IJ, Essink-bot ML, Hop WC, Schalm SW. Subclinical hepatic encephalopathy impairs daily functioning. Hepatology. 1998;28:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 355] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 10. | Wein C, Koch H, Popp B, Oehler G, Schauder P. Minimal hepatic encephalopathy impairs fitness to drive. Hepatology. 2004;39:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 210] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 11. | Iguchi H, Kato KI, Ibayashi H. Melatonin serum levels and metabolic clearance rate in patients with liver cirrhosis. J Clin Endocrinol Metab. 1982;54:1025-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Steindl PE, Finn B, Bendok B, Rothke S, Zee PC, Blei AT. Disruption of the diurnal rhythm of plasma melatonin in cirrhosis. Ann Intern Med. 1995;123:274-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 109] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Garfinkel D, Zisapel N. Liver cirrhosis and circadian rhythm. Ann Intern Med. 1996;125:154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Córdoba J, Cabrera J, Lataif L, Penev P, Zee P, Blei AT. High prevalence of sleep disturbance in cirrhosis. Hepatology. 1998;27:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 191] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Schomerus H, Hamster W, Blunck H, Reinhard U, Mayer K, Dölle W. Latent portasystemic encephalopathy. I. Nature of cerebral functional defects and their effect on fitness to drive. Dig Dis Sci. 1981;26:622-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 162] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Schomerus H, Hamster W. Quality of life in cirrhotics with minimal hepatic encephalopathy. Metab Brain Dis. 2001;16:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 150] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Srivastava A, Mehta R, Rothke SP, Rademaker AW, Blei AT. Fitness to drive in patients with cirrhosis and portal-systemic shunting: a pilot study evaluating driving performance. J Hepatol. 1994;21:1023-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Amodio P, Del Piccolo F, Marchetti P, Angeli P, Iemmolo R, Caregaro L, Merkel C, Gerunda G, Gatta A. Clinical features and survivial of cirrhotic patients with subclinical cognitive alterations detected by the number connection test and computerized psychometric tests. Hepatology. 1999;29:1662-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 176] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Hartmann IJ, Groeneweg M, Quero JC, Beijeman SJ, de Man RA, Hop WC, Schalm SW. The prognostic significance of subclinical hepatic encephalopathy. Am J Gastroenterol. 2000;95:2029-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 159] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Tarter RE, Arria AM, Carra J, Van Thiel DH. Memory impairments concomitant with nonalcoholic cirrhosis. Int J Neurosci. 1987;32:853-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Weissenborn K, Heidenreich S, Giewekemeyer K, Rückert N, Hecker H. Memory function in early hepatic encephalopathy. J Hepatol. 2003;39:320-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Gilberstadt SJ, Gilberstadt H, Zieve L, Buegel B, Collier RO, McClain CJ. Psychomotor performance defects in cirrhotic patients without overt encephalopathy. Arch Intern Med. 1980;140:519-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 90] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Rehnström S, Simert G, Hansson JA, Johnson G, Vang J. Chronic hepatic encephalopathy. A psychometrical study. Scand J Gastroenterol. 1977;12:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Rikkers L, Jenko P, Rudman D, Freides D. Subclinical hepatic encephalopathy: detection, prevalence, and relationship to nitrogen metabolism. Gastroenterology. 1978;75:462-469. [PubMed] |
| 25. | Tarter RE, Hegedus AM, Van Thiel DH, Schade RR, Gavaler JS, Starzl TE. Nonalcoholic cirrhosis associated with neuropsychological dysfunction in the absence of overt evidence of hepatic encephalopathy. Gastroenterology. 1984;86:1421-1427. [PubMed] |
| 26. | McCrea M, Cordoba J, Vessey G, Blei AT, Randolph C. Neuropsychological characterization and detection of subclinical hepatic encephalopathy. Arch Neurol. 1996;53:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 114] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Weissenborn K, Heidenreich S, Ennen J, Rückert N, Hecker H. Attention deficits in minimal hepatic encephalopathy. Metab Brain Dis. 2001;16:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Schomerus H, Hamster W. Neuropsychological aspects of portal-systemic encephalopathy. Metab Brain Dis. 1998;13:361-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Jover R, Compañy L, Gutiérrez A, Lorente M, Zapater P, Poveda MJ, Such J, Pascual S, Palazón JM, Carnicer F. Clinical significance of extrapyramidal signs in patients with cirrhosis. J Hepatol. 2005;42:659-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Bengtsson F, Nobin A, Falck B, Gage FH, Jeppsson B. Portacaval shunt in the rat: selective alterations in behavior and brain serotonin. Pharmacol Biochem Behav. 1986;24:1611-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 53] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Bergqvist PB, Werner ER, Apelqvist G, Bugge M, Wachter H, Bengtsson F. Brain biopterin metabolism in chronic experimental hepatic encephalopathy. Metab Brain Dis. 1995;10:143-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Martin JR. Discordant motor activity levels of portacaval-shunted rats in runway and swim tests. Behav Neurosci. 1986;100:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Steindl PE, Coy DL, Finn B, Zee PC, Blei AT. A low-protein diet ameliorates disrupted diurnal locomotor activity in rats after portacaval anastomosis. Am J Physiol. 1996;271:G555-G560. [PubMed] |
| 34. | Theander B, Apelqvist G, Bugge M, Andersson G, Hindfelt B, Bengtsson F. Gender and diurnal effects on specific open-field behavioral patterns in the portacaval shunted rat. Metab Brain Dis. 1997;12:47-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Erceg S, Monfort P, Hernández-Viadel M, Rodrigo R, Montoliu C, Felipo V. Oral administration of sildenafil restores learning ability in rats with hyperammonemia and with portacaval shunts. Hepatology. 2005;41:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 36. | Erceg S, Monfort P, Hernandez-Viadel M, Llansola M, Montoliu C, Felipo V. Restoration of learning ability in hyperammonemic rats by increasing extracellular cGMP in brain. Brain Res. 2005;1036:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Aguilar MA, Miñarro J, Felipo V. Chronic moderate hyperammonemia impairs active and passive avoidance behavior and conditional discrimination learning in rats. Exp Neurol. 2000;161:704-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Cauli O, Llansola M, Erceg S, Felipo V. Hypolocomotion in rats with chronic liver failure is due to increased glutamate and activation of metabotropic glutamate receptors in substantia nigra. J Hepatol. 2006;45:654-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Monfort P, Muñoz MD, ElAyadi A, Kosenko E, Felipo V. Effects of hyperammonemia and liver failure on glutamatergic neurotransmission. Metab Brain Dis. 2002;17:237-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Felipo V, Miñana MD, Grisolía S. Long-term ingestion of ammonium increases acetylglutamate and urea levels without affecting the amount of carbamoyl-phosphate synthase. Eur J Biochem. 1988;176:567-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Azorín I, Miñana MD, Felipo V, Grisolía S. A simple animal model of hyperammonemia. Hepatology. 1989;10:311-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Hermenegildo C, Montoliu C, Llansola M, Muñoz MD, Gaztelu JM, Miñana MD, Felipo V. Chronic hyperammonemia impairs the glutamate-nitric oxide-cyclic GMP pathway in cerebellar neurons in culture and in the rat in vivo. Eur J Neurosci. 1998;10:3201-3209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 154] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 43. | Monfort P, Corbalán R, Martinez L, López-Talavera J, Córdoba J, Felipo V. Altered content and modulation of soluble guanylate cyclase in the cerebellum of rats with portacaval anastomosis. Neuroscience. 2001;104:1119-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Corbalán R, Chatauret N, Behrends S, Butterworth RF, Felipo V. Region selective alterations of soluble guanylate cyclase content and modulation in brain of cirrhotic patients. Hepatology. 2002;36:1155-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | Apelqvist G, Hindfelt B, Andersson G, Bengtsson F. Diurnal and gender effects by chronic portacaval shunting in rats on spontaneous locomotor and rearing activities in an open-field. Behav Brain Res. 1998;93:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Apelqvist G, Hindfelt B, Andersson G, Bengtsson F. Altered adaptive behaviour expressed in an open-field paradigm in experimental hepatic encephalopathy. Behav Brain Res. 1999;106:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Lozeva V, Valjakka A, Lecklin A, Olkkonen H, Hippeläinen M, Itkonen M, Plumed C, Tuomisto L. Effects of the histamine H(1) receptor blocker, pyrilamine, on spontaneous locomotor activity of rats with long-term portacaval anastomosis. Hepatology. 2000;31:336-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Martin JR, Oettinger R, Bättig K. Behavioral effects of experimental portacaval anastomosis measured in Dashiell and radial tunnel maze configurations. Physiol Behav. 1986;38:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 49. | Burkhard PR, Delavelle J, Du Pasquier R, Spahr L. Chronic parkinsonism associated with cirrhosis: a distinct subset of acquired hepatocerebral degeneration. Arch Neurol. 2003;60:521-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 173] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 50. | Kulisevsky J, Pujol J, Balanzó J, Junqué C, Deus J, Capdevilla A, Villanueva C. Pallidal hyperintensity on magnetic resonance imaging in cirrhotic patients: clinical correlations. Hepatology. 1992;16:1382-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Spahr L, Butterworth RF, Fontaine S, Bui L, Therrien G, Milette PC, Lebrun LH, Zayed J, Leblanc A, Pomier-Layrargues G. Increased blood manganese in cirrhotic patients: relationship to pallidal magnetic resonance signal hyperintensity and neurological symptoms. Hepatology. 1996;24:1116-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 190] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 52. | Lockwood AH, Yap EW, Rhoades HM, Wong WH. Altered cerebral blood flow and glucose metabolism in patients with liver disease and minimal encephalopathy. J Cereb Blood Flow Metab. 1991;11:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 107] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 53. | Weissenborn K, Kolbe H. The basal ganglia and portal-systemic encephalopathy. Metab Brain Dis. 1998;13:261-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Turski L, Klockgether T, Turski W, Schwarz M, Sontag KH. Substantia nigra and motor control in the rat: effect of intranigral alpha-kainate and gamma-D-glutamylaminomethylsulphonate on motility. Brain Res. 1987;424:37-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Millan MH, Wardley-Smith B, Halsey MJ, Meldrum BS. Studies on the role of the NMDA receptor in the substantia nigra pars reticulata and entopeduncular nucleus in the development of the high pressure neurological syndrome in rats. Exp Brain Res. 1989;78:174-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Trevitt T, Carlson B, Correa M, Keene A, Morales M, Salamone JD. Interactions between dopamine D1 receptors and gamma-aminobutyric acid mechanisms in substantia nigra pars reticulata of the rat: neurochemical and behavioral studies. Psychopharmacology (Berl). 2002;159:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Kaur S, Starr MS. Differential effects of intrastriatal and intranigral injections of glutamate antagonists on motor behaviour in the reserpine-treated rat. Neuroscience. 1997;76:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |