Published online Dec 28, 2006. doi: 10.3748/wjg.v12.i48.7725
Revised: August 28, 2006
Accepted: October 10, 2006
Published online: December 28, 2006
The liver is an essential player in the pathway of coagulation in both primary and secondary haemostasis. Only von Willebrand factor is not synthetised by the liver, thus liver failure is associated with impairment of coagulation. However, recently it has been shown that the delicate balance between pro and antithrombotic factors synthetised by the liver might be reset to a lower level in patients with chronic liver disease. Therefore, these patients might not be really anticoagulated in stable condition and bleeding may be caused only when additional factors, such as infections, supervene. Portal hypertension plays an important role in coagulopathy in liver disease, reducing the number of circulating platelets, but platelet function and secretion of thrombopoietin have been also shown to be impaired in patients with liver disease. Vitamin K deficiency may coexist, so that abnormal clotting factors are produced due to lack of gamma carboxylation. Moreover during liver failure, there is a reduced capacity to clear activated haemostatic proteins and protein inhibitor complexes from the circulation. Usually therapy for coagulation disorders in liver disease is needed only during bleeding or before invasive procedures. When end stage liver disease occurs, liver transplantation is the only treatment available, which can restore normal haemostasis, and correct genetic clotting defects, such as haemophilia or factor V Leiden mutation. During liver transplantation haemorrage may occur due to the pre-existing hypocoagulable state, the collateral circulation caused by portal hypertension and increased fibrinolysis which occurs during this surgery.
- Citation: Senzolo M, Burra P, Cholongitas E, Burroughs A. New insights into the coagulopathy of liver disease and liver transplantation. World J Gastroenterol 2006; 12(48): 7725-7736
- URL: https://www.wjgnet.com/1007-9327/full/v12/i48/7725.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i48.7725
The liver plays several key roles in blood coagulation being involved in both primary and secondary hemostasis[1]. It is the site of synthesis of all coagulation factors and their inhibitors except for von Willebrand factor (vWf)[2]. Liver damage is commonly associated with impairment of coagulation, when liver reserve is poor. The hemostatic system is in a delicate balance between prothrombotic and antithrombotic processes, aiming to prevent excessive blood loss from injured vessels and to prevent spontaneous thrombosis. Liver failure is accompanied by multiple changes in the hemostatic system, because of reduced plasma levels of procoagulative and anticoagulative clotting factors synthesised by hepatocytes and sinusoidal cells[3]. Vitamin K deficiency may coexist, so that abnormal clotting factors are produced due to lack of gamma carboxylation. Moreover during liver failure, there is a reduced capacity to clear activated hemostatic proteins and protein inhibitor complexes from the circulation. Thus the global effect of liver disease with regard to hemostasis is complex, so that patients with advanced liver disease can experience severe bleeding or even thrombotic complications (Table 1). Finally, when marked portal hypertension develops with collateral circulation and secondary splenomegaly, thrombocytopenia develops due to splenic sequestration. However, thrombocytopenia may also be due to decreased hepatic thrombopoietin synthesis. There is also impaired platelet function. These hemostatic abnormalities do not always lead to spontaneous bleeding, but the onset of complications of cirrhosis such as variceal bleeding or infection/sepsis may lead to worsening of the coagulation status. The presence of a consumptive coagulopathy other than secondary to sepsis or other pre-disposing causes is disputed.
| Favoring hemorrage | Favoring thrombosis |
| Low platelet count | |
| Impaired platelet function and platelet-vessel wall interaction | Elevated levels of factors VIII and vWf |
| Enhanced platelet inhibition by nitric oxide (NO) and prostacyclin | Decreased levels of protein C, protein S, antithrormbin |
| III, α2-antiplasmin | |
| Decreased levels coagulation factors (II, V, VII, IX, X, XI) | Macroglobulin |
| Quantitative and qualitative abnormalities of fibrinogen | Heparin cofactor II elevated |
| Low level of α2-antiplasmin, TAFI, histidine-rich-glycoprotein | Decreased levels of plasminogen |
| levels of tPA, with small increase of PAI-1 levels |
Usually therapy for coagulation disorders in liver disease is needed only during bleeding or before invasive procedures. When end stage liver disease occurs, liver transplantation is the only treatment available, which can restore normal hemostasis, and correct genetic clotting defects, such as hemophilia or factor V Leiden mutation. During liver transplantation hemorrage may occur due to the pre-existing hypocoagulable state, the collateral circulation caused by portal hypertension and increased fibrinolysis which occurs during this surgery.
The liver is the site of synthesis of fibrinogen and factors II, V, VII, IX, X, XI and XII[4]. Von Willebrand factor (vWf) is synthetised by the endothelium[5]. Factor VIII is synthetised mainly by the hepatic, but also non hepatic sinusoidal endothelial cells[6-8], thus the plasma concentration of factor VIII is not decreased with liver disease, and may be even increased, as many chronic liver diseases are associated with chronic inflammation[9]. Factor VIII is high in fulminant hepatic failure and low in disseminated intravascular coagulation (DIC)[10] but this differential diagnosis is seldom an issue in clinical practice.
Vitamin K is an essential cofactor for the production of biologically active forms of the coagulation factors II, VII, IX and X. When γ-carboxylation is impaired due to deficiency or antagonism of vitamin K, inert precurcors are synthesised, (known as Proteins Induced by Vitamin K Absence [PIVKA]) and released into the blood stream[11]. The clinical significance of these precursors is not clear. In the case of prothrombin, a specific and sensitive immunoassay for this incomplete PIVKA prothrombin detects changes before conventional coagulation tests[12]. In cholestasis, vitamin K absorption from the small intestine is reduced due to decreased bile salt production. It can be corrected by vitamin K 10 mg daily for 24-48 h, but in parenchymal liver disease as there is a decreased synthesis of coagulation factors, there is no improvement with vitamin K[13]. However, 25% of patients with acute liver injury have a subclinical deficit of vitamin K which improves with parenteral administration of vit K[14].
In acute liver failure, plasma concentration of coagulation factors first those with the shortest half life, factor V and VII (12 h and 4-6 h respectively), and factors II, VII and X subsequently[15]. Factor VIII, together with vWf is usually elevated. The differential effects on clotting factor concentrations during acute liver failure occur because high cytokine concentrations increasing tissue factor (TF) which activates factors II, V, VII, X, whereas any thrombin generated is inhibited by antithrombin III, preventing activation of factors VIII, XI and consequently XI, thus preserving their plasma levels[9].
Prothrombin gene mutation (G20210A) is the most common thrombophilic cause of portal vein thrombosis without cirrhosis (22% of cases)[16]. In contrast, factor V Leiden mutation is common thrombophilic disorder (20%) associated with hepatic vein thrombosis in Western countries[17].
Plasma concentration of vWf is increased in patients with acute liver failure, due to increased synthesis as an acute phase protein in response to tissue injury[18-20] and also endothelial dysfunction secondary to endotoxemia[5]. In chronic liver disease, endothelial shear stress related to portal hypertension may also contribute to the high plasma levels of vWf via a nitric oxide stimulus[21]. A correlation between severity of liver disease and vWf plasma antigen levels has been documented.
Plasma fibrinogen is an acute-phase reactant, and remains normal or increased in patients with liver disease[22]. Low concentrations due to decreased synthesis, yet above 100 mg/dL, are only seen with very severe liver disease[23]. However the high fibrinogen concentrations found in patients with chronic hepatitis, cholestatic jaundice and hepatocellular carcinoma, do not result in increased clot formation as most is a non-functional fibrinogen present in 60%-70%: there are abnormal α chains and a higher sialic acid content[24]. This is due to an increased activity of sialil-transferase in immature hepatocytes generated during hepatic injury; this results in an abnormal thrombin time (TT), despite an almost normal PT and PTT, with an apparent normal or raised concentration of fibrinogen.
Abnormalities in both number and function of platelets are common in liver disease and contribute to the impaired hemostasis.
About one third of patients with chronic liver disease develop thrombocytopenia, (70.000-90.000 × 109/L), which worsens in parallel with disease progression associated with increased platelet sequestration due to hypersplenism[25-27].
Thrombocytopenia appears not to be associated with an increased risk of bleeding from esophageal varices or other sites, although there are only few studies evaluating this, but it is correlated with blood loss during surgery[28]. A higher spleen diameter/platelet count ratio is highly predictive for the presence of esophageal varices in patients with liver cirrhosis[29].
Splenic sequestration versus other causes of thrombocytopenia in cirrhosis has been recently evaluated by comparing platelet number in extrahepatic portal hypertension, to that of cirrhosis in patients having a similar sized spleen. There is less severe thrombocytopenia in the non-cirrhotic patient[30]. Synthetic function of the liver is essential for platelet production via thrombopoietin (TPO), which regulates platelet production in the bone marrow[31]. Although TPO increases in patients with thrombocytopenia due to a homeostatic response[32], this occurs to a lesser degree with severe or chronic liver disease, than in patients with a normal liver[33]. Lower TPO mRNA levels in cirrhotic liver tissue[34] have been shown, confirming impaired TPO synthesis. In addition, a low platelet production from the bone marrow in cirrhotic patients has been shown[35].
Hepatitis C virus (HCV)[36] acute viral infection, alcohol abuse and folate deficiency can all result in some myelosuppression[37] further lowering platelet counts. Thrombocytopenia may also be contributed to by immune mediated mechanisms due to an increase production from B cells of antibodies binding platelet surface antigen GPIIb-IIIa and GPIb/I, shown in viral related cirrhosis B and C[38], primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC)[39].
Platelet aggregation in response to ADP, arachidonic acid, collagen and thrombin is subnormal, probably due to a defective signal transduction mechanism[19]. Intrinsic defects including an abnormal arachidonic acid membrane content[40] and abnormal plasma factors[41] have also been shown to contribute to platelet function abnormalities. In cholestatic liver diseases there often is a normal or hypercoagulable state evaluated by thromboelastography[42] and there can be normal or hyperactive platelet function when assessed by platelet function assay (PFA-100) closure time and flow cytometric study of receptors[43]. When platelet number is too low, both cytometry and aggregation studies may be difficult to interpret. Thromboelastography which is a global test of clot formation and dissolution measures both platelet function and number by the maximum amplitude (ma) parameter[44] which can be used to assess platelet function.
Splenectomy is generally contra-indicated in patients with liver cirrhosis, because of the high mortality rate and a risk of secondary portal vein thrombosis , which leads to bleeding from esophageal-gastric varices and more difficult surgery during subsequent liver transplant[45]. Splenic embolization with 30%-50% reduction in flow can normalize or significantly improve platelet number in some cirrhotics[46] and it is sometimes used before embolisation of hepatocellular carinoma or interferon therapy for viral hepatitis. Insertion of transjugular intrahepatic portosystemic shunt (TIPS) increases, but it does not to normalize platelet number[47,48].
Antithrombin III (ATIII) is a non-vitamin K-dependent glycoprotein synthesised by the liver and endothelium[49]. In liver diseases, concentration falls due to reduced synthesis and/or increased consumption due to hyperfibrinolysis[50]. Usually the ATIII deficit is mild and thrombotic complications are very rare[51]. ATIII replacement does not correct hyperfibrinolysis in cirrhotic patients.
Proteins C and S are vitamin K dependent glycoproteins synthesised mainly by hepatocytes[52]. During acute or chronic liver disease, their concentrations decrease concomitantly with the other coagulation factors, but usually not below 20% of normal[53]. Genetic deficiency of protein C is rare in the general population and portal vein thrombosis[54], but is found in 20% patients with Budd-Chiari syndrome (BCS). In patients with liver disease who also have genetic deficiency, plasma concentration is often lower than 20%. When there is severe liver disease, it can be difficult to exclude coexistent genetic deficiency as levels may be very low, due to very depressed synthesis[17]. In this situation a concomitant finding of a normal level of factor II and protein C/factor VII ratio, can help to confirm a coexistent genetic deficit[55]. Genetic deficiency of protein S is extremely rare, but accounts for 7% of patients with BCS or portal vein thrombosis (PVT), especially in series from Asia[56].
All the proteins involved in fibrinolysis, except for tPA and PAI-1 are synthesized in the liver. Reduced plasma levels of plasminogen[57], α2-antiplasmin, histidine-rich glycoprotein (HRG)[58], factor XIII[59], and thrombin-activable fibrinolysis inhibitor (TAFI)[57] are found in cirrhosis. Conversely tPA levels are increased in liver disease, due to decreased clearance, whereas it’s inhibitor PAI-1 is normal or only slightly increased in plasma. The inhibitor concentrations are insufficient to counteract the increase in tPA, accounting for increased fibrinolysis[60]. In contrast, in acute liver failure, there are high levels of the acute phase reactant PAI-1 leading to a shift towards hypofibrinolysis[61].
Hyperfibrinolysis is correlated with the severity of liver dysfunction in cirrhosis as assessed by Child-Pugh score[62]. Ascitic fluid has increased fibrinolytic activity: up to 20 liters are reabsorbed daily, with fibrinolysis being correlated with endotoxin levels[63]. Increased levels of D-dimers, prothrombin fragments 1+2 (F1+2) fibrin degradation products and plasmin-α2-antiplasmin complexes are found[64]. Many studies using different methodologies demonstrate hyperfibrinolysis (thromboelastography[65], diluted whole blood clot lysis assay[66] and euglobin clot lysis time[67]) . TAFI is decreased by an average of 26% in cirrhosis and by 50% in acute liver failure[68,69]. However there is some controversy as regarding hyperfibrinolytic activity in cirrhotics as not all studies have confirmed this.
Interestingly, patients with cholestatic liver diseases, are characterized by a normal or hypercoagulable state: higher PAI-1 concentrations are seen compared to other etiologies, balancing the increased tPA activity. This results in less hyperfibrinolysis in the reperfusion phase during liver transplantation, and antifibrinolytic therapy is not usually administered[70]. Thus the clinical issue is whether cirrhotic patients when under “stress” (e.g. during infection, during surgery or during bleeding) exhibit the increased fibrinolysis, resulting in an increased bleeding tendency, which is not manifest in laboratory terms when patients are stable.
DIC is characterized by intravascular fibrin deposition due to activation of the clotting cascade, which overwhelms the anticoagulation pathway. Secondarily there is consumption of coagulation factors and platelets, associated with secondary fibrinolysis, causing an increased bleeding tendency[71].
Low grade DIC and the hemostatic abnormalities which are present in cirrhotics; they share common laboratory features, ie a prolonged PT and PTT, low fibrinogen level, elevated fibrin-degradation product and D-dimer and thrombocytopenia[72-74]. Thus differential diagnosis by laboratory means alone may be confounding. Early reports linked chronic liver disease to low grade DIC, ascribing the latter to accelerated fibrinolysis. However, the presence of DIC in liver cirrhosis is disputed[75]. Although DIC-like laboratory abnormalities (so called “pseudo-DIC) are observed, autopsy studies in cirrhotics have shown little evidence for fibrin deposition and clinically manifest DIC is very rare[72].
More highly sensitive tests such as quantification of proteolytic cleavage products of the coagulation reaction ie fibrinopeptide A, F1+2, and fibrinolysis reactions (fibrin D-dimer, high molecular weight fibrin/fibrinogen complexes or soluble fibrin), demonstrate an abnormal profile called accelerated intravascular coagulation and fibrinolysis phenomenon (AICF)[75]. The studies to date demonstrate AICF in about 30% of cirrhotics, depending on the severity of liver disease[65].
However, Ben Ari et al analyzed 52 patients with stable liver disease for F1+2 thrombin-antithrombin III complex (TAT) and D-dimer levels which were no different from controls, yet TEG studies were able to detect hyperfibrinolysis. AICF may be important in the portal venous system, as this phenomenon is more pronounced there than in systemic blood[65]. This could be related to higher levels of endotoxemia in portal blood, which can trigger release of IL6 and TNF-alfa thus activating intravascular coagulation[76].
In cirrhosis, plasma levels coagulation factors are indicators of hepatic synthesis and thus of liver function. A prolonged PT, which is not corrected by intravenous vitamin K administration 10 mg daily for 2 d, helps differentiate vitamin K deficiency from parenchymal liver diseases[13]. PT is part of the Child-Pugh score, which is the most commonly used prognostic score assessing the severity of liver disease[77]. Recently the MELD score which incorporates INR has been used to allocate priority for liver transplantation in the USA based on estimated probability of death within 3 mo[78].
Determination of individual coagulation factors adds little prognostic information to measuring PT or INR in cirrhosis. A multivariate analysis of prognostic factors in cirrhotic patients showed that the level of factor VII was an independent predictor factor of survival: factor VII < 34% was predictive of a mortality in 93%[79].
In acute liver failure, the Clichy criteria indicate poor prognosis and need for liver transplantation, when factor V is below 20% in patients aged ≤ 30 or below 30% associated with age ≥ 30[80]. Factor V has less prognostic value in acetaminophen-induced fulminant hepatic failure[81].
In the King’s College criteria in acetaminophen-induced liver failure, PT ≥ 100 s is a prognostic indicator on its own for liver transplantation independent of the grade of coma. In patients with non-acetaminophen induced ALF, PT ≥ 50 s together with two of the following criteria: age < 10 > 40 years, drug toxicity, interval between jaundice and encephalopathy onset > 7 d and serum biliubin > 300 μmol/L are indications of poor prognosis and for liver transplantation[82].
Thrombotic complications can paradoxically occur in cirrhotic patients even if clinically an increased risk of haemorrhage is considered. Despite prolonged coagulation tests, these patients cannot be viewed as being “anti-coagulated”. Wanless et al has put forward portal and hepatic vein thrombosis as cause of disease progression in cirrhotic patients. Hepatic and portal vein thrombosis was found in at least 70% of explanted livers, and 36% were associated with regions of confluent fibrosis (focal parenchymal extinction)[83], which is a histological correlate of chronic thrombosis.
Portal vein thrombosis complicates liver cirrhosis between 0.6% to 15% of cases, leading to worsening of liver function, development of ascites and occasionally mesenteric infarction[84]. In these patients early anticoagula-tion is indicated and has been shown to recanalise the splanchnic veins in about 50% of cases and prevent the extension of the thrombus without causing increased haemorragic complications[85].
In BCS, even if a prothrombotic cause is not identified, anticoagulation should be started immediately after diagnosis, as many genetic prothrombic defects remain yet to be identified and acquired disorders, common in BCS, may be difficult to diagnose, such as polycythaemia rubra vera or paroxysmal nocturnal hemoglobinuria (PNH). Early anticoagulation ameliorates prognosis. Anticoagulation therapy should continue even after liver transplantation because of the high rate of recurrence and thrombotic complications after OLT, and also because other prothrombotic disorders may exist alongside the diagnosed protein deficiencies[17,86].
The risk of deep vein thrombosis and pulmonary embolism is not well documented in cirrhotics, yet is reported[87]. Patients with cholestatic disease often exhibit a procoagulant state demonstrated by TEG, may be prone thrombosis, but this has not been studied[42]. No guidelines are available for the management of thrombotic complications and neither for prevention of embolic phenomena for example following atrial fibrillation in cirrhotic patients.
The role played by coagulation defects in the occurrence of bleeding in cirrhosis is still unclear. This is particularly due to the difficulty (and cost) in measuring procoagulant and anticoagulant activities, and assessing the balance between the two (Table 1). In addition there are very few tests which reflect coagulation in vivo. Recently generation of thrombin has been explored in vitro in cirrhotic patients and found to be normal. In this study, a resetting of the coagulation and anticoagulation system at a lower level was postulated, because during liver disease both procoagulant and anticoagulant pathways are affected in a parallel manner. However, the in vitro technique has some drawbacks, the major one being that platelets are substituted by phospholipids[3].
Minor signs of bleeding tendency are common, such as gum bleeding and epistaxis, but major bleeding can be encountered. The role of hemostatic abnormalities in variceal bleeding is not clear. Hyperfibrinolysis has been shown to be linked but not necessary causal to an increased risk of variceal bleeding, in a cohort of 61 cirrhotics. Higher levels of fibrinogen degradation products were associated with a greater risk of variceal bleeding compared to patients without (odds ratio = 8), but Child-Pugh score and endoscopic characteristics of varices remain the most important prognostic factors[88]. Recently the role of infection and endogenous heparin-like substances demonstrated by TEG has been evaluated in variceal bleeding. Infection may be a trigger factor for bleeding[89] and both infection and heparin-like substances may be mechanisms responsible for the persistence of bleeding in some[90]. TEG, which is a quick and reliable method to assess clot formation and lysis[44], also allows detection of heparin-like substances. Studies from our group have shown worsening coagulation during infection due to low molecular weight heparin-like substances detected by TEG[91].
Historically, PT and platelet count have been used to assess the risk of bleeding prior to invasive procedures. Cirrhotic patients have increased mortality and morbidity during surgery[92], mainly due to increased bleeding in 60% of cases[93,94]. Early studies linked PT to surgical risk (PT prolongation > 1.5 and > 2.5 s associated with 47% and 87% mortality respectively)[95], hence platelet count < 50.000/mm3 and PT > 3 s have been considered relative contraindications to elective surgery[94]. In addition, portal hypertension and collateral veins increase the risk of bleeding during surgical dissection.
Hyperfibrinolysis[96] and clotting activation, due to increase tPA levels have been described in patients undergoing liver resection[97]. However, another study performed in patients undergoing laparoscopic liver biopsy failed to demonstrate any correlation between the risk of bleeding evaluated at the hepatic puncture site and coagulation tests, so that the degree of injury may be the important factor[98].
Liver biopsy is widely used diagnostically and to grade the severity of liver disease or fibrosis. Moreover it is an essential tool after liver transplantation to diagnose rejection and other causes of graft dysfunction. Bleeding complications occur in 0.35%-0.5%, leading to mortality in 0.1%[99]. Despite the evidence that there were no threshold abnormalities of clotting tests associated with risk of bleeding during laparoscopic liver biopsy, INR and platelet count are considered essential to evaluate the bleeding risk for percutaneous liver biopsy[100]. An audit from the British Society of Gastroenterology (BSG) performed in 1991 showed a doubling of bleeding risk in patients with INR ≥ 1.5, but that only 7.1% of the bleeding occurred with INR greater than 1.5, and 90% occurred with a INR ≤ 1.3[101]. A cut off for platelet count is difficult to justify from the literature. Most textbooks in the UK and BSG guidelines, require platelet count above 80.000/mm3[13] whereas a survey form the Mayo Clinic suggested 50.000/ mm3 as a cut off[102]. Current recommendations state that a percutaneous liver biopsy can be done safely without support with platelet counts are above 60.000/mm3[100]. Burroughs et al advocated evaluating the use of bleeding time to assess the risk of bleeding for percutaneous liver biopsy[103], but this is not routine in clinical practice. If clotting parameters are outside stipulated ranges, a transjugular liver biopsy can be performed more safely, without plasma or platelet therapy[104]. A plugged liver biopsy is also said to be safer, but it may cause greater risk of bleeding in hypocoagulable patients[99].
During minor procedures such as thoracentesis, paracentesis or lumbar puncture performed in patients with liver disease, there are no firm guidelines as to the hemostatic threshold for performing these tests. A contraindication to the procedure is clinically evident DIC or fibrinolysis[105].
The overall cumulative incidence of infection in cirrhotic patients is estimated to be at least 30%[106], and is possibly associated with increased risk of variceal bleeding[89]. Infection is associated with early rebleeding and increased mortality[107,108]. Prophylactic antibiotic therapy has led to less early rebleeding and better control of bleeding, in a randomized study[109].
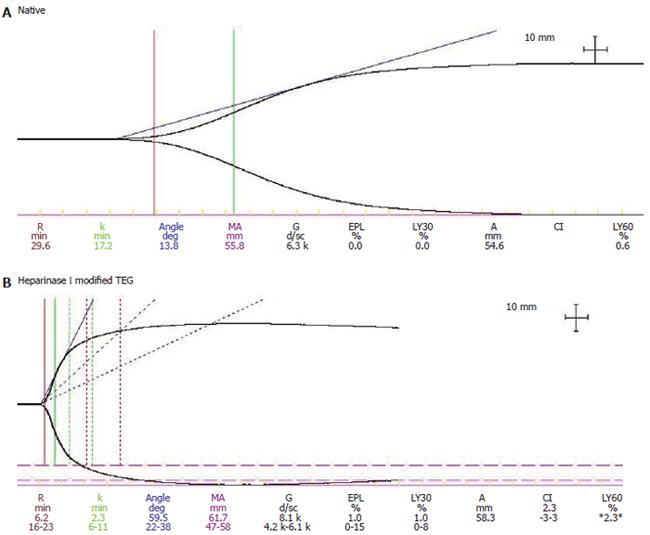
Using TEG, 20 cirrhotic patients who experienced early rebleeding were found to have worsening TEG parameters the day before rebleeding[90], Moreover patients with bacterial infection have worse TEG parameters, which are corrected in vitro by heparinase I, which cleaves heparin-like substances[91] (Figure 1A and 1B). The presence of heparin like substances is associated in some with increased antiXa activity[110]. Heparin-like substances have been detected hours after variceal bleeding in cirrhotic patients[111]. Based on this evidence the hypothesis has been postulated that endotoxins and inflammation due to infection can release heparinoids from the endothelium and mast cells[91]. Moreover sepsis can cause impairment of platelet function, decreasing platelet number and aggregability, due to increase NO production[112].
Therapy for hemostatic abnormalities of liver disease is needed only during variceal bleeding, surgery or before invasive procedures. Intravenous vitamin K injecton of 10 mg daily for 24-48 h can replace vitamin K deficiency[13].
Fresh frozen plasma (FFP) contains all the clotting factors and can correct the laboratory finding of an elevated PT effectively, but this correction depends on the volume and the baseline abnormality of PT. Whether this correction of the PT results in increasing hemostasis has yet to be proven. In addition, correction is short term (24-48 h), depending on the half-life of the clotting factors (especially factor VII)[71]. A common indication for FFP infusion is the presence of persistent bleeding in patients with INR ≥ 2 or PT prolongation greater than 4 s[113]. In surgical or invasive procedures 50% of the normal PT (ie INR of 2) is a target for replacement therapy, and for neurological procedures such as intracranial pressure monitoring during liver failure, 80% of normal PT range (ie an INR of about 1.2-1.3)[113]. During massive blood transfusion, to avoid dilutional decrease of clotting factors for every 2 units of blood, 1 of FFP is typically given[114]. To increase the activity of clotting factor by 1%-2% a dose of 1 mL FFP/kg of body weight is necessary[115]. Because of the high volume required, adequate replacement is difficult both in cirrhotic patients (intravascular plasma volume is already expanded and ascites may be present), and ALF, (increasing plasma volume can lead to increases in intracerebral pressure). Moreover, the short half-life requires infusion every 6-12 h[10]. In patients with INR > 1.5, FFP is given (12-15 mL/kg) before liver biopsy, but there is no evidence base for this. Transjugular biopsy should be used in patients with coagulopathy not sufficiently corrected with FFP.
Platelet transfusion, one unit every 10 kg is typically administered, and platelet count should be checked 1 h after the infusion[116]. However no correlation between amelioration of bleeding time, increase in platelet count, and enhanced hemostasis has been shown[100].
Cryoprecipitate contains factors VIII, fibrinogen, vWf, fibronectin and XIII. Because of the small volumes (30-50 mL/U/10 kg) required[116], it can be useful in liver cirrhosis and ALF, but it lacks some coagulation factors and may worsen the imbalance already present in patients with liver disease.
Desmopressin (1-deamino-8-D-arginine vasopressin [DDAVP]), an analogue of the antidiuretic hormone, increases plasma level of factor VIII and vWf, probably by increasing the release from endothelial storage sites[117]. It can improve bleeding time, enhancing primary hemostasis at the dose of 0.3 μg/kg in patients with liver failure[118]. However a randomised trial associating terlipressin and DDAVP in patients with variceal bleeding, demonstrated no difference in control of bleeding and maybe a worsening of the terlipressin action in the DDVAP group[119]. In a recent randomized trial, DDVAP failed to decrease blood loss during hepatic resection, despite increase of factor VIII and vWf[120]. ATIII infusion is not routinely recommended.
Recombinant activated factor VII (rFVIIa) was first developed for the treatment of patients with hemophilia A and B who developed inhibitors. It may have promising role in the treatment of coagulation disorders in liver disease[121]. A single dose of recombinant factor VIIa has been shown to correct prolonged PT in a dose-dependent manner in non-bleeding cirrhotic patients[122]. A randomized study using rFVIIa in 71 patients undergoing laparoscopic liver biopsy found no differences in liver bleeding time. Two complications occured in the rFVIIa group (1 DIC and 1 PVT)[123]. In ALF, rFVIIa may be useful to normalize PT in the setting of intracranial pressure monitoring, as only a small volume of infusion is required. During variceal bleeding in a randomized trial, a modest reduction of early rebleeding rate was observed in a subgroup of Child B and C patients after rFVIIa infusion, although, no difference in control of bleeding or transfusion was shown overall[124]. Another report described initial hemostasis after infusion of rFVIIa in 10 patients with variceal bleeding, but 6 experienced early rebleeding and all of them died, illustrating the short interval of action of this drug[125]. In a cohort of 8 patients with acute variceal bleeding uncontrolled with endoscopic and medical therapy, rFVIIa administration achieved hemostasis in 25% after a single dose[126].
Safety of rFVIIa, especially about the possible prothrombotic effect or triggering of DIC, still has to be assessed in large studies in patients with liver disease[127].
Orthotopic liver transplantation (OLT) is the only cure for end stage liver disease. Improvements in operative management, surgical techniques and graft preservation have contributed to a significant reduction in transfusion requirements during the last decade[128]. However, blood losses are highly variable, and correlate in most studies with a higher mortality, poor graft function and risk of infections[129]. In current practice a significant proportion of patients receive no blood during surgery.
Most studies failed to define factors related to bleeding, including preoperative coagulation tests or markers of fibrinolysis during liver transplantation[130-132], with the exception of the collateral circulation due to portal hypertension and previous abdominal surgery [133].
Hemostatic abnormalities during liver transplantation are divided according to the surgical phases which are traditionally: pre-anhepatic phase, anhepatic phase and post reperfusion phase and post operative period.
The first operative stage is characterized by extensive surgical trauma, resulting from dissection of adhesions in the abdominal cavity and transection of many collateral vessels. Usually during this phase, mild coagulation abnormalities occur and the blood losses are mainly correlated with the surgical technique and the baseline hypocoagulable state[133], but etiology of liver disease can also influence the blood product requirement. Hypercoagulability has been demonstrated in patients with hepatocellular carcinoma as well as cholestatic cirrhosis (PBC, PSC). The PBC and PSC patients have a hypercoagulable state by TEG[42] and less fibrinolytic activity during OLT than other aetiologies[134], suggesting that in these patients antifibrinolytic drugs should not be used. Moreover in pediatric liver transplantation for biliary atresia, plasma studies showed less coagulation abnormalities during OLT compared to other etiologies[135]. Enhanced fibrinolytic activity contributes to blood loss in the pre-anhepatic phase in only 10%-20% of patients[136].
During this phase no important surgical blood loss is seen because appropriate vessels are clamped. However, bleeding can occur due to hemostatic changes in this phase. Despite impairment of synthetic and clearance function, early studies failed to show dramatic changes in PT and PTT[129,137]. However, hyperfibrinolysis has been demonstrated in many studies, due to net increase in tPA derived from endothelial cells; this tPA is not cleared due to the absence of the liver at this time[138]. The presence of an active fibrinolytic process has been demonstrated by simultaneous decrease of α2-antiplasmin and plasminogen activity, and a concomitant increase in fibrin and fibrinogen degradation products[139]. Use of rFVIIa has been tried in patients with severe coagulopathy (INR 5.7 and 6.9). Moderate bleeding was still reported during surgery, but 1 patient developed hepatic artery thrombosis after transplant[140]. Studies which evaluated coagulation factors during OLT after rFVIIa infusion showed a sharp increase of thrombin generation, PT and PTT, but no amelioration of fibrinolysis[141,142].
Reperfusion of the liver is a crucial point of the operation and leads to profound coagulation abnormalities. Within minutes after reperfusion, uncontrollable diffuse bleeding may occur in some patients[143].
Trapping of platelets in the graft may play a role in the bleeding tendency. Experimental studies have shown a 55% gradient in platelet count between arterial and venous blood flow in the new liver. Moreover, some alteration in the bleeding time and platelet function and aggregation have been demonstrated[144]. Signs of DIC after graft reperfusion have been shown by some investigators, mainly correlated with poor quality of the transplanted organ[145].
Increase in fibrinolysis has been implicated as the most important and significant phenomenon responsible for bleeding during liver transplantation. It usually subsides within 60 min after graft reperfusion, but in donor livers with poor function, a sustained increased fibrinolytic response can be seen[146].
After reperfusion, release of heparin or heparin-like substances has been shown in 25%-95% of cases[147]. Protamine sulphate (50 mg) has been used in vivo to antagonize this effect. One study has confirmed the presence of heparin-like compounds using heparinase I-modified TEG, which cleaves heparin and heparan sulphate. Increased blood product requirement was correlated with the presence of heparin like effects in TEG traces. However a baseline heparin-like effect has recently been found before reperfusion in patients undergoing liver transplantation not receiving heparin[148].
Antifibrinolytic therapy is used during liver transplantation to reduce blood loss, time of surgery and fibrinolytic activity. Aprotinin is a serine protease inhibitor which antagonizes various proteases[149]. Aprotinin also has anti-inflammatory and anti-oxidant effects which might also be of benefit. Widespread use of aprotinin is not recommended because of the risk of anaphylactic reactions, renal dysfunction and stroke[150], which has been also recently stressed by a multicentre study on 4357 patients undergoing cardiac surgery[151], but this is not been reported nor studied in liver transplantation.
Epsilon aminocaproic acid (EACA) interferes with plasminogen binding to fibrin and thus EACA inhibits the conversion of plasminogen to plasmin[152]. In the only prospective randomized trial, it was shown to reverse TEG fibrinolysis, and reduce blood cell transfusion, without causing thrombotic complications. However this reduction was not statistically significant compared to controls[153]. Similar to EACA, tranexamic acid inhibits fibrinolysis, but it is 6 to 10 times more potent than EACA[154]. Recent trials have shown that at a dose of 2 mg/kg per hour, tranexamic acid reduces fibrinolysis and blood loss. However different doses have been used in other studies without clearcut effects[155-157].
The routine use of coagulation monitoring during liver transplantation is common place. Usually TEG is used, a point of care rapid method to assess the whole coagulation process. It provides the basis of a rational approach to the use of blood component therapy or pharmacologic intervention, but it does not help in addressing blood transfusion per se[44]. Recently TEG was used to monitor postoperative coagulation in patients undergoing hepatic resection for living related liver transplantation. In these patients, a hypercoagulable state correlated with the risk of developing thrombotic complications after surgery[158].
Thrombocytopenia is common in the early post-operative period, mainly due to platelet activation and consumption following graft reperfusion[159]. Thrombocytopenia is common in the early post-operative period, mainly due to platelet activation and consumption following graft reperfusion[159], and if liver function restores thrombocytopenia subsides a few day after OLT. Following normal synthetic function of the liver, thrombopoietin levels increase significantly on the first day, following by immature bone marrow megakariocites after 3 d and new circulating platelets after 5 d Normalization of platelet count can be seen after 14 d[160]. Peak of TPO level correlates with the pre-OLT platelet count. Levels of bilirubin, cold ischemia time or episodes of rejection do not influence TPO levels[161]. Persistence of thrombocytopenia can be seen in some patients, which can be ascribed to persistent splenomegaly in some[162].
S- Editor Wang J L- Editor Chiarioni G E- Editor Bai SH
| 1. | Lisman T, Leebeek FW, de Groot PG. Haemostatic abnormalities in patients with liver disease. J Hepatol. 2002;37:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 143] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Rapaport SI. Coagulation problems in liver disease. Blood Coagul Fibrinolysis. 2000;11 Suppl 1:S69-S74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Tripodi A, Salerno F, Chantarangkul V, Clerici M, Cazzaniga M, Primignani M, Mannuccio Mannucci P. Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology. 2005;41:553-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 498] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 4. | Kelly DA, Summerfield JA. Hemostasis in liver disease. Semin Liver Dis. 1987;7:182-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Ferro D, Quintarelli C, Lattuada A, Leo R, Alessandroni M, Mannucci PM, Violi F. High plasma levels of von Willebrand factor as a marker of endothelial perturbation in cirrhosis: relationship to endotoxemia. Hepatology. 1996;23:1377-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 119] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Wion KL, Kelly D, Summerfield JA, Tuddenham EG, Lawn RM. Distribution of factor VIII mRNA and antigen in human liver and other tissues. Nature. 1985;317:726-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 189] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Hollestelle MJ, Geertzen HG, Straatsburg IH, van Gulik TM, van Mourik JA. Factor VIII expression in liver disease. Thromb Haemost. 2004;91:267-275. [PubMed] |
| 8. | Hollestelle MJ, Thinnes T, Crain K, Stiko A, Kruijt JK, van Berkel TJ, Loskutoff DJ, van Mourik JA. Tissue distribution of factor VIII gene expression in vivo--a closer look. Thromb Haemost. 2001;86:855-861. [PubMed] |
| 9. | Kerr R. New insights into haemostasis in liver failure. Blood Coagul Fibrinolysis. 2003;14 Suppl 1:S43-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Mueller MM, Bomke B, Seifried E. Fresh frozen plasma in patients with disseminated intravascular coagulation or in patients with liver diseases. Thromb Res. 2002;107 Suppl 1:S9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Blanchard RA, Furie BC, Jorgensen M, Kruger SF, Furie B. Acquired vitamin K-dependent carboxylation deficiency in liver disease. N Engl J Med. 1981;305:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 137] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Belle M, Brebant R, Guinet R, Leclercq M. Production of a new monoclonal antibody specific to human des-gamma-carboxyprothrombin in the presence of calcium ions. Application to the development of a sensitive ELISA-test. J Immunoassay. 1995;16:213-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Sherlock S, Dooley J. The haematololgy of liver disease. Diseases of the liver and biliary system. 2002;47-64. |
| 14. | Pereira SP, Rowbotham D, Fitt S, Shearer MJ, Wendon J, Williams R. Pharmacokinetics and efficacy of oral versus intravenous mixed-micellar phylloquinone (vitamin K1) in severe acute liver disease. J Hepatol. 2005;42:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Kerr R, Newsome P, Germain L, Thomson E, Dawson P, Stirling D, Ludlam CA. Effects of acute liver injury on blood coagulation. J Thromb Haemost. 2003;1:754-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Amitrano L, Brancaccio V, Guardascione MA, Margaglione M, Iannaccone L, D'Andrea G, Marmo R, Ames PR, Balzano A. Inherited coagulation disorders in cirrhotic patients with portal vein thrombosis. Hepatology. 2000;31:345-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 172] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Senzolo M, Cholongitas EC, Patch D, Burroughs AK. Update on the classification, assessment of prognosis and therapy of Budd-Chiari syndrome. Nat Clin Pract Gastroenterol Hepatol. 2005;2:182-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Baruch Y, Neubauer K, Ritzel A, Wilfling T, Lorf T, Ramadori G. Von Willebrand gene expression in damaged human liver. Hepatogastroenterology. 2004;51:684-688. [PubMed] |
| 19. | Escolar G, Cases A, Viñas M, Pino M, Calls J, Cirera I, Ordinas A. Evaluation of acquired platelet dysfunctions in uremic and cirrhotic patients using the platelet function analyzer (PFA-100 ): influence of hematocrit elevation. Haematologica. 1999;84:614-619. [PubMed] |
| 20. | Kelly DA, Tuddenham EG. Haemostatic problems in liver disease. Gut. 1986;27:339-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Albornoz L, Alvarez D, Otaso JC, Gadano A, Salviú J, Gerona S, Sorroche P, Villamil A, Mastai R. Von Willebrand factor could be an index of endothelial dysfunction in patients with cirrhosis: relationship to degree of liver failure and nitric oxide levels. J Hepatol. 1999;30:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Lechner K, Niessner H, Thaler E. Coagulation abnormalities in liver disease. Semin Thromb Hemost. 1977;4:40-56. [PubMed] |
| 23. | Dymock IW, Tucker JS, Woolf IL, Poller L, Thomson JM. Coagulation studies as a prognostic index in acute liver failure. Br J Haematol. 1975;29:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 44] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Francis JL, Armstrong DJ. Fibrinogen-bound sialic acid levels in the dysfibrinogenaemia of liver disease. Haemostasis. 1982;11:215-222. [PubMed] |
| 25. | Noguchi H, Hirai K, Aoki Y, Sakata K, Tanikawa K. Changes in platelet kinetics after a partial splenic arterial embolization in cirrhotic patients with hypersplenism. Hepatology. 1995;22:1682-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Sohma Y, Akahori H, Seki N, Hori T, Ogami K, Kato T, Shimada Y, Kawamura K, Miyazaki H. Molecular cloning and chromosomal localization of the human thrombopoietin gene. FEBS Lett. 1994;353:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 163] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Yanaga K, Tzakis AG, Shimada M, Campbell WE, Marsh JW, Stieber AC, Makowka L, Todo S, Gordon RD, Iwatsuki S. Reversal of hypersplenism following orthotopic liver transplantation. Ann Surg. 1989;210:180-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Clavien PA, Camargo CA, Croxford R, Langer B, Levy GA, Greig PD. Definition and classification of negative outcomes in solid organ transplantation. Application in liver transplantation. Ann Surg. 1994;220:109-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 280] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Giannini E, Botta F, Borro P, Risso D, Romagnoli P, Fasoli A, Mele MR, Testa E, Mansi C, Savarino V. Platelet count/spleen diameter ratio: proposal and validation of a non-invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut. 2003;52:1200-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 298] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 30. | Robson SC, Kahn D, Kruskal J, Bird AR, Kirsch RE. Disordered hemostasis in extrahepatic portal hypertension. Hepatology. 1993;18:853-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Panasiuk A, Prokopowicz D, Zak J, Panasiuk B. Reticulated platelets as a marker of megakaryopoiesis in liver cirrhosis; relation to thrombopoietin and hepatocyte growth factor serum concentration. Hepatogastroenterology. 2004;51:1124-1128. [PubMed] |
| 32. | Jelkmann W. The role of the liver in the production of thrombopoietin compared with erythropoietin. Eur J Gastroenterol Hepatol. 2001;13:791-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Kitano K, Shimodaira S, Ito T, Ichikawa N, Kodaira H, Kohara Y, Ueno M, Tahara T, Kato T, Ishida F. Liver cirrhosis with marked thrombocytopenia and highly elevated serum thrombopoietin levels. Int J Hematol. 1999;70:52-55. [PubMed] |
| 34. | Martin TG 3rd, Somberg KA, Meng YG, Cohen RL, Heid CA, de Sauvage FJ, Shuman MA. Thrombopoietin levels in patients with cirrhosis before and after orthotopic liver transplantation. Ann Intern Med. 1997;127:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510-5514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4392] [Cited by in RCA: 4724] [Article Influence: 157.5] [Reference Citation Analysis (0)] |
| 36. | Nagamine T, Ohtuka T, Takehara K, Arai T, Takagi H, Mori M. Thrombocytopenia associated with hepatitis C viral infection. J Hepatol. 1996;24:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 147] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 37. | Peck-Radosavljevic M. Thrombocytopenia in liver disease. Can J Gastroenterol. 2000;14 Suppl D:60D-66D. [PubMed] |
| 38. | Kajihara M, Kato S, Okazaki Y, Kawakami Y, Ishii H, Ikeda Y, Kuwana M. A role of autoantibody-mediated platelet destruction in thrombocytopenia in patients with cirrhosis. Hepatology. 2003;37:1267-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Feistauer SM, Penner E, Mayr WR, Panzer S. Target platelet antigens of autoantibodies in patients with primary biliary cirrhosis. Hepatology. 1997;25:1343-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Laffi G, Marra F, Gresele P, Romagnoli P, Palermo A, Bartolini O, Simoni A, Orlandi L, Selli ML, Nenci GG. Evidence for a storage pool defect in platelets from cirrhotic patients with defective aggregation. Gastroenterology. 1992;103:641-646. [PubMed] |
| 41. | Younger HM, Hadoke PW, Dillon JF, Hayes PC. Platelet function in cirrhosis and the role of humoral factors. Eur J Gastroenterol Hepatol. 1997;9:989-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Ben-Ari Z, Panagou M, Patch D, Bates S, Osman E, Pasi J, Burroughs A. Hypercoagulability in patients with primary biliary cirrhosis and primary sclerosing cholangitis evaluated by thrombelastography. J Hepatol. 1997;26:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 133] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 43. | Pihusch R, Rank A, Göhring P, Pihusch M, Hiller E, Beuers U. Platelet function rather than plasmatic coagulation explains hypercoagulable state in cholestatic liver disease. J Hepatol. 2002;37:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Salooja N, Perry DJ. Thrombelastography. Blood Coagul Fibrinolysis. 2001;12:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 235] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 45. | Bolognesi M, Merkel C, Sacerdoti D, Nava V, Gatta A. Role of spleen enlargement in cirrhosis with portal hypertension. Dig Liver Dis. 2002;34:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 174] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 46. | N'Kontchou G, Seror O, Bourcier V, Mohand D, Ajavon Y, Castera L, Grando-Lemaire V, Ganne-Carrie N, Sellier N, Trinchet JC. Partial splenic embolization in patients with cirrhosis: efficacy, tolerance and long-term outcome in 32 patients. Eur J Gastroenterol Hepatol. 2005;17:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 47. | Jabbour N, Zajko A, Orons P, Irish W, Fung JJ, Selby RR. Does transjugular intrahepatic portosystemic shunt (TIPS) resolve thrombocytopenia associated with cirrhosis. Dig Dis Sci. 1998;43:2459-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Karasu Z, Gurakar A, Kerwin B, Hulagu S, Jazzar A, McFadden R, Nour B, Sebastian A, Cassidy F, Stokes K. Effect of transjugular intrahepatic portosystemic shunt on thrombocytopenia associated with cirrhosis. Dig Dis Sci. 2000;45:1971-1976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Schipper HG, ten Cate JW. Antithrombin III transfusion in patients with hepatic cirrhosis. Br J Haematol. 1982;52:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Liebman HA, McGehee WG, Patch MJ, Feinstein DI. Severe depression of antithrombin III associated with disseminated intravascular coagulation in women with fatty liver of pregnancy. Ann Intern Med. 1983;98:330-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Carmassi F, Morale M, De Negri F, Carrai M. Modulation of hemostatic balance with antithrombin III replacement therapy in a case of liver cirrhosis associated with recurrent venous thrombosis. J Mol Med (Berl). 1995;73:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Fair DS, Marlar RA. Biosynthesis and secretion of factor VII, protein C, protein S, and the Protein C inhibitor from a human hepatoma cell line. Blood. 1986;67:64-70. [PubMed] |
| 53. | Mannucci PM, Vigano S. Deficiencies of protein C, an inhibitor of blood coagulation. Lancet. 1982;2:463-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 150] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 54. | Primignani M, Martinelli I, Bucciarelli P, Battaglioli T, Reati R, Fabris F, Dell'era A, Pappalardo E, Mannucci PM. Risk factors for thrombophilia in extrahepatic portal vein obstruction. Hepatology. 2005;41:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 55. | Minnema MC, Janssen HL, Niermeijer P, de Man RA. Budd-Chiari syndrome: combination of genetic defects and the use of oral contraceptives leading to hypercoagulability. J Hepatol. 2000;33:509-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Bhattacharyya M, Makharia G, Kannan M, Ahmed RP, Gupta PK, Saxena R. Inherited prothrombotic defects in Budd-Chiari syndrome and portal vein thrombosis: a study from North India. Am J Clin Pathol. 2004;121:844-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 57. | Stein SF, Harker LA. Kinetic and functional studies of platelets, fibrinogen, and plasminogen in patients with hepatic cirrhosis. J Lab Clin Med. 1982;99:217-230. [PubMed] |
| 58. | Leebeek FW, Kluft C, Knot EA, De Maat MP. Histidine-rich glycoprotein is elevated in mild liver cirrhosis and decreased in moderate and severe liver cirrhosis. J Lab Clin Med. 1989;113:493-497. [PubMed] |
| 59. | Biland L, Duckert F, Prisender S, Nyman D. Quantitative estimation of coagulation factors in liver disease. The diagnostic and prognostic value of factor XIII, factor V and plasminogen. Thromb Haemost. 1978;39:646-656. [PubMed] |
| 60. | Hersch SL, Kunelis T, Francis RB. The pathogenesis of accelerated fibrinolysis in liver cirrhosis: a critical role for tissue plasminogen activator inhibitor. Blood. 1987;69:1315-1319. [PubMed] |
| 61. | Pernambuco JR, Langley PG, Hughes RD, Izumi S, Williams R. Activation of the fibrinolytic system in patients with fulminant liver failure. Hepatology. 1993;18:1350-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 62. | Hu KQ, Yu AS, Tiyyagura L, Redeker AG, Reynolds TB. Hyperfibrinolytic activity in hospitalized cirrhotic patients in a referral liver unit. Am J Gastroenterol. 2001;96:1581-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 63. | Agarwal S, Joyner KA, Swaim MW. Ascites fluid as a possible origin for hyperfibrinolysis in advanced liver disease. Am J Gastroenterol. 2000;95:3218-3224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 64. | Páramo JA, Rocha E. Hemostasis in advanced liver disease. Semin Thromb Hemost. 1993;19:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Ben-Ari Z, Osman E, Hutton RA, Burroughs AK. Disseminated intravascular coagulation in liver cirrhosis: fact or fiction. Am J Gastroenterol. 1999;94:2977-2982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 66. | Comp PC, Jacocks RM, Rubenstein C, Radcliffe R. A lysine-absorbable plasminogen activator is elevated in conditions associated with increased fibrinolytic activity. J Lab Clin Med. 1981;97:637-645. [PubMed] |
| 67. | Francis RB, Feinstein DI. Clinical significance of accelerated fibrinolysis in liver disease. Haemostasis. 1984;14:460-465. [PubMed] |
| 68. | Lisman T, Leebeek FW, Mosnier LO, Bouma BN, Meijers JC, Janssen HL, Nieuwenhuis HK, De Groot PG. Thrombin-activatable fibrinolysis inhibitor deficiency in cirrhosis is not associated with increased plasma fibrinolysis. Gastroenterology. 2001;121:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 207] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 69. | Colucci M, Binetti BM, Branca MG, Clerici C, Morelli A, Semeraro N, Gresele P. Deficiency of thrombin activatable fibrinolysis inhibitor in cirrhosis is associated with increased plasma fibrinolysis. Hepatology. 2003;38:230-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 70. | Segal H, Cottam S, Potter D, Hunt BJ. Coagulation and fibrinolysis in primary biliary cirrhosis compared with other liver disease and during orthotopic liver transplantation. Hepatology. 1997;25:683-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 71. | Amitrano L, Guardascione MA, Brancaccio V, Balzano A. Coagulation disorders in liver disease. Semin Liver Dis. 2002;22:83-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 245] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 72. | Carr JM. Disseminated intravascular coagulation in cirrhosis. Hepatology. 1989;10:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 73. | Violi F, Ferro D, Basili S, Saliola M, Quintarelli C, Alessandri C, Cordova C. Association between low-grade disseminated intravascular coagulation and endotoxemia in patients with liver cirrhosis. Gastroenterology. 1995;109:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 74. | Kemkes-Matthes B, Bleyl H, Matthes KJ. Coagulation activation in liver diseases. Thromb Res. 1991;64:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 75. | Joist JH. AICF and DIC in liver cirrhosis: expressions of a hypercoagulable state. Am J Gastroenterol. 1999;94:2801-2803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 76. | Basili S, Ferro D, Violi F. Endotoxaemia, hyperfibrinolysis, and bleeding in cirrhosis. Lancet. 1999;353:1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 77. | Botero RC, Lucey MR. Organ allocation: model for end-stage liver disease, Child-Turcotte-Pugh, Mayo risk score, or something else. Clin Liver Dis. 2003;7:715-27, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 78. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3676] [Article Influence: 153.2] [Reference Citation Analysis (0)] |
| 79. | Violi F, Ferro D, Basili S, Cimminiello C, Saliola M, Vezza E, Cordova C. Prognostic value of clotting and fibrinolytic systems in a follow-up of 165 liver cirrhotic patients. CALC Group. Hepatology. 1995;22:96-100. [PubMed] |
| 80. | Bismuth H, Samuel D, Castaing D, Adam R, Saliba F, Johann M, Azoulay D, Ducot B, Chiche L. Orthotopic liver transplantation in fulminant and subfulminant hepatitis. The Paul Brousse experience. Ann Surg. 1995;222:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 205] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 81. | Pauwels A, Mostefa-Kara N, Florent C, Lévy VG. Emergency liver transplantation for acute liver failure. Evaluation of London and Clichy criteria. J Hepatol. 1993;17:124-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 122] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | O'Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439-445. [PubMed] |
| 83. | Wanless IR, Wong F, Blendis LM, Greig P, Heathcote EJ, Levy G. Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension. Hepatology. 1995;21:1238-1247. [PubMed] |
| 84. | Amitrano L, Guardascione MA, Brancaccio V, Margaglione M, Manguso F, Iannaccone L, Grandone E, Balzano A. Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis. J Hepatol. 2004;40:736-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 393] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 85. | Francoz C, Belghiti J, Vilgrain V, Sommacale D, Paradis V, Condat B, Denninger MH, Sauvanet A, Valla D, Durand F. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut. 2005;54:691-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 392] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 86. | Mentha G, Giostra E, Majno PE, Bechstein WO, Neuhaus P, O'Grady J, Praseedom RK, Burroughs AK, Le Treut YP, Kirkegaard P. Liver transplantation for Budd-Chiari syndrome: A European study on 248 patients from 51 centres. J Hepatol. 2006;44:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 87. | Espiritu JD. Pulmonary embolism in a patient with coagulopathy from end-stage liver disease. Chest. 2000;117:924-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 88. | Violl F, Basili S, Ferro D, Quintarelli C, Alessandril C, Cordova C. Association between high values of D-dimer and tissue-plasminogen activator activity and first gastrointestinal bleeding in cirrhotic patients. CALC Group. Thromb Haemost. 1996;76:177-183. [PubMed] |
| 89. | Goulis J, Patch D, Burroughs AK. Bacterial infection in the pathogenesis of variceal bleeding. Lancet. 1999;353:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 179] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 90. | Chau TN, Chan YW, Patch D, Tokunaga S, Greenslade L, Burroughs AK. Thrombelastographic changes and early rebleeding in cirrhotic patients with variceal bleeding. Gut. 1998;43:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 91. | Montalto P, Vlachogiannakos J, Cox DJ, Pastacaldi S, Patch D, Burroughs AK. Bacterial infection in cirrhosis impairs coagulation by a heparin effect: a prospective study. J Hepatol. 2002;37:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 143] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 92. | Perkins L, Jeffries M, Patel T. Utility of preoperative scores for predicting morbidity after cholecystectomy in patients with cirrhosis. Clin Gastroenterol Hepatol. 2004;2:1123-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 93. | Patel T. Surgery in the patient with liver disease. Mayo Clin Proc. 1999;74:593-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 94. | Friedman LS. The risk of surgery in patients with liver disease. Hepatology. 1999;29:1617-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 176] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 95. | Garrison RN, Cryer HM, Howard DA, Polk HC. Clarification of risk factors for abdominal operations in patients with hepatic cirrhosis. Ann Surg. 1984;199:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 314] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 96. | Tsuji K, Eguchi Y, Kodama M. Postoperative hypercoagulable state followed by hyperfibrinolysis related to wound healing after hepatic resection. J Am Coll Surg. 1996;183:230-238. [PubMed] |
| 97. | Meijer C, Wiezer MJ, Hack CE, Boelens PG, Wedel NI, Meijer S, Nijveldt RJ, Statius Muller MG, Wiggers T, Zoetmulder FA. Coagulopathy following major liver resection: the effect of rBPI21 and the role of decreased synthesis of regulating proteins by the liver. Shock. 2001;15:261-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 98. | Ewe K. Bleeding after liver biopsy does not correlate with indices of peripheral coagulation. Dig Dis Sci. 1981;26:388-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 240] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 99. | Burroughs AK, Dagher L. Liver biopsy. Gastroenterological Endoscopy. New york: Thieme 2002; 252-259. |
| 100. | Grant A, Neuberger J. Guidelines on the use of liver biopsy in clinical practice. British Society of Gastroenterology. Gut. 1999;45 Suppl 4:IV1-IV11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 186] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 101. | Gilmore IT, Burroughs A, Murray-Lyon IM, Williams R, Jenkins D, Hopkins A. Indications, methods, and outcomes of percutaneous liver biopsy in England and Wales: an audit by the British Society of Gastroenterology and the Royal College of Physicians of London. Gut. 1995;36:437-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 282] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 102. | McGill DB, Rakela J, Zinsmeister AR, Ott BJ. A 21-year experience with major hemorrhage after percutaneous liver biopsy. Gastroenterology. 1990;99:1396-1400. [PubMed] |
| 103. | Blake JC, Sprengers D, Grech P, McCormick PA, McIntyre N, Burroughs AK. Bleeding time in patients with hepatic cirrhosis. BMJ. 1990;301:12-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 104. | Papatheodoridis GV, Patch D, Watkinson A, Tibballs J, Burroughs AK. Transjugular liver biopsy in the 1990s: a 2-year audit. Aliment Pharmacol Ther. 1999;13:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 105. | Runyon BA. Paracentesis of ascitic fluid. A safe procedure. Arch Intern Med. 1986;146:2259-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 138] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 106. | Bernard B, Grangé JD, Khac EN, Amiot X, Opolon P, Poynard T. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: a meta-analysis. Hepatology. 1999;29:1655-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 461] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 107. | Goulis J, Armonis A, Patch D, Sabin C, Greenslade L, Burroughs AK. Bacterial infection is independently associated with failure to control bleeding in cirrhotic patients with gastrointestinal hemorrhage. Hepatology. 1998;27:1207-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 256] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 108. | Bernard B, Cadranel JF, Valla D, Escolano S, Jarlier V, Opolon P. Prognostic significance of bacterial infection in bleeding cirrhotic patients: a prospective study. Gastroenterology. 1995;108:1828-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 187] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 109. | Hou MC, Lin HC, Liu TT, Kuo BI, Lee FY, Chang FY, Lee SD. Antibiotic prophylaxis after endoscopic therapy prevents rebleeding in acute variceal hemorrhage: a randomized trial. Hepatology. 2004;39:746-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 245] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 110. | Zambruni A, Thalheimer U, Coppell J, Riddell A, Mancuso A, Leandro G, Perry D, Burroughs AK. Endogenous heparin-like activity detected by anti-Xa assay in infected cirrhotic and non-cirrhotic patients. Scand J Gastroenterol. 2004;39:830-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 111. | Thalheimer U, Triantos C, Samonakis D, Patch D, Burroughs AK, Riddell A, Perry D. Endogenous heparinoids in acute variceal bleeding. Gut. 2005;54:310-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 112. | Thalheimer U, Triantos CK, Samonakis DN, Patch D, Burroughs AK. Infection, coagulation, and variceal bleeding in cirrhosis. Gut. 2005;54:556-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 219] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 113. | Everson GT. A hepatologist's perspective on the management of coagulation disorders before liver transplantation. Liver Transpl Surg. 1997;3:646-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 114. | Martin DJ, Lucas CE, Ledgerwood AM, Hoschner J, McGonigal MD, Grabow D. Fresh frozen plasma supplement to massive red blood cell transfusion. Ann Surg. 1985;202:505-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 39] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 115. | Maltz GS, Siegel JE, Carson JL. Hematologic management of gastrointestinal bleeding. Gastroenterol Clin North Am. 2000;29:169-87, vii. [PubMed] |
| 116. | Günter P. Practice guidelines for blood component therapy. Anesthesiology. 1996;85:1219-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 117. | Mannucci PM, Canciani MT, Rota L, Donovan BS. Response of factor VIII/von Willebrand factor to DDAVP in healthy subjects and patients with haemophilia A and von Willebrand's disease. Br J Haematol. 1981;47:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 195] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 118. | Burroughs AK, Matthews K, Qadiri M, Thomas N, Kernoff P, Tuddenham E, McIntyre N. Desmopressin and bleeding time in patients with cirrhosis. Br Med J (Clin Res Ed). 1985;291:1377-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 65] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 119. | de Franchis R, Arcidiacono PG, Carpinelli L, Andreoni B, Cestari L, Brunati S, Zambelli A, Battaglia G, Mannucci PM. Randomized controlled trial of desmopressin plus terlipressin vs. terlipressin alone for the treatment of acute variceal hemorrhage in cirrhotic patients: a multicenter, double-blind study. New Italian Endoscopic Club. Hepatology. 1993;18:1102-1107. [PubMed] |
| 120. | Wong AY, Irwin MG, Hui TW, Fung SK, Fan ST, Ma ES. Desmopressin does not decrease blood loss and transfusion requirements in patients undergoing hepatectomy. Can J Anaesth. 2003;50:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 121. | Caldwell SH, Chang C, Macik BG. Recombinant activated factor VII (rFVIIa) as a hemostatic agent in liver disease: a break from convention in need of controlled trials. Hepatology. 2004;39:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 122. | Bernstein DE, Jeffers L, Erhardtsen E, Reddy KR, Glazer S, Squiban P, Bech R, Hedner U, Schiff ER. Recombinant factor VIIa corrects prothrombin time in cirrhotic patients: a preliminary study. Gastroenterology. 1997;113:1930-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 163] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 123. | Jeffers L, Chalasani N, Balart L, Pyrsopoulos N, Erhardtsen E. Safety and efficacy of recombinant factor VIIa in patients with liver disease undergoing laparoscopic liver biopsy. Gastroenterology. 2002;123:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 105] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 124. | Bosch J, Thabut D, Bendtsen F, D'Amico G, Albillos A, González Abraldes J, Fabricius S, Erhardtsen E, de Franchis R. Recombinant factor VIIa for upper gastrointestinal bleeding in patients with cirrhosis: a randomized, double-blind trial. Gastroenterology. 2004;127:1123-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 260] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 125. | Ejlersen E, Melsen T, Ingerslev J, Andreasen RB, Vilstrup H. Recombinant activated factor VII (rFVIIa) acutely normalizes prothrombin time in patients with cirrhosis during bleeding from oesophageal varices. Scand J Gastroenterol. 2001;36:1081-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 126. | Romero-Castro R, Jimenez-Saenz M, Pellicer-Bautista F, Gomez-Parra M, Argüelles Arias F, Guerrero-Aznar MD, Sendon-Perez A, Herrerias-Gutierrez JM. Recombinant-activated factor VII as hemostatic therapy in eight cases of severe hemorrhage from esophageal varices. Clin Gastroenterol Hepatol. 2004;2:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 127. | Shapiro SS. Treating thrombosis in the 21st century. N Engl J Med. 2003;349:1762-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 128. | Kang YG, Martin DJ, Marquez J, Lewis JH, Bontempo FA, Shaw BW, Starzl TE, Winter PM. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth Analg. 1985;64:888-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 368] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 129. | Palomo Sanchez JC, Jimenez C, Moreno Gonzalez E, Garcia I, Palma F, Loinaz C, Gonzalez Ghamorro A. Effects of intraoperative blood transfusion on postoperative complications and survival after orthotopic liver transplantation. Hepatogastroenterology. 1998;45:1026-1033. [PubMed] |
| 130. | Steib A, Freys G, Lehmann C, Meyer C, Mahoudeau G. Intraoperative blood losses and transfusion requirements during adult liver transplantation remain difficult to predict. Can J Anaesth. 2001;48:1075-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 115] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 131. | Steib A, Gengenwin N, Freys G, Boudjema K, Levy S, Otteni JC. Predictive factors of hyperfibrinolytic activity during liver transplantation in cirrhotic patients. Br J Anaesth. 1994;73:645-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 132. | Hendriks HG, van der Meer J, Klompmaker IJ, Choudhury N, Hagenaars JA, Porte RJ, de Kam PJ, Slooff MJ, de Wolf JT. Blood loss in orthotopic liver transplantation: a retrospective analysis of transfusion requirements and the effects of autotransfusion of cell saver blood in 164 consecutive patients. Blood Coagul Fibrinolysis. 2000;11 Suppl 1:S87-S93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 133. | Kirby RM, McMaster P, Clements D, Hubscher SG, Angrisani L, Sealey M, Gunson BK, Salt PJ, Buckels JA, Adams DH. Orthotopic liver transplantation: postoperative complications and their management. Br J Surg. 1987;74:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 63] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 134. | Palareti G, Legnani C, Maccaferri M, Gozzetti G, Mazziotti A, Martinelli G, Zanello M, Sama C, Coccheri S. Coagulation and fibrinolysis in orthotopic liver transplantation: role of the recipient's disease and use of antithrombin III concentrates. S. Orsola Working Group on Liver Transplantation. Haemostasis. 1991;21:68-76. [PubMed] |
| 135. | Carlier M, Van Obbergh LJ, Veyckemans F, de Kock M, de Beys CC, Lavenne-Pardonge E, Moulin D, Otte JB. Hemostasis in children undergoing liver transplantation. Semin Thromb Hemost. 1993;19:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 136. | Kang Y. Coagulation and liver transplantation: current concepts. Liver Transpl Surg. 1997;3:465-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 137. | Lewis JH, Bontempo FA, Awad SA, Kang YG, Kiss JE, Ragni MV, Spero JA, Starzl TE. Liver transplantation: intraoperative changes in coagulation factors in 100 first transplants. Hepatology. 1989;9:710-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 138. | Loskutoff DJ, Edgington TE. Synthesis of a fibrinolytic activator and inhibitor by endothelial cells. Proc Natl Acad Sci USA. 1977;74:3903-3907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 246] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 139. | Emeis JJ, van den Hoogen CM, Jense D. Hepatic clearance of tissue-type plasminogen activator in rats. Thromb Haemost. 1985;54:661-664. [PubMed] |
| 140. | Kaliciński P, Kamiński A, Drewniak T, Ismail H, Szymczak M, Markiewicz M, Lukasiewicz H. Quick correction of hemostasis in two patients with fulminant liver failure undergoing liver transplantation by recombinant activated factor VII. Transplant Proc. 1999;31:378-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 141. | Hendriks HG, Meijer K, de Wolf JT, Porte RJ, Klompmaker IJ, Lip H, Slooff MJ, van der Meer J. Effects of recombinant activated factor VII on coagulation measured by thromboelastography in liver transplantation. Blood Coagul Fibrinolysis. 2002;13:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 142. | Meijer K, Hendriks HG, De Wolf JT, Klompmaker IJ, Lisman T, Hagenaars AA, Slooff MJ, Porte RJ, van der Meer J. Recombinant factor VIIa in orthotopic liver transplantation: influence on parameters of coagulation and fibrinolysis. Blood Coagul Fibrinolysis. 2003;14:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 143. | Porte RJ. Coagulation and fibrinolysis in orthotopic liver transplantation: current views and insights. Semin Thromb Hemost. 1993;19:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 66] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 144. | Hutchison DE, Genton E, Porter KA, Daloze PM, Huguet C, Brettschneider L, Groth CG, Starzl TE. Platelet changes following clinical and experimental hepatic homotransplantation. Arch Surg. 1968;97:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 145. | Porte RJ, Knot EA, Bontempo FA. Hemostasis in liver transplantation. Gastroenterology. 1989;97:488-501. [PubMed] |
| 146. | Bakker CM, Blankensteijn JD, Schlejen P, Porte RJ, Gomes MJ, Lampe HI, Stibbe J, Terpstra OT. The effects of long-term graft preservation on intraoperative hemostatic changes in liver transplantation. A comparison between orthotopic and heterotopic transplantation in the pig. HPB Surg. 1994;7:265-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 147. | Moriau M, Kestens PJ, Masure R. Heparin and antifibrinolytic agents during experimental hepatectomy and liver transplantation. Pathol Eur. 1969;4:172-182. [PubMed] |
| 148. | Harding SA, Mallett SV, Peachey TD, Cox DJ. Use of heparinase modified thrombelastography in liver transplantation. Br J Anaesth. 1997;78:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 149. | Shore-Lesserson L. Point-of-care coagulation monitoring for cardiovascular patients: past and present. J Cardiothorac Vasc Anesth. 2002;16:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 150. | Vater Y, Levy A, Martay K, Hunter C, Weinbroum AA. Adjuvant drugs for end-stage liver failure and transplantation. Med Sci Monit. 2004;10:RA77-RA88. [PubMed] |
| 151. | Mangano DT, Tudor IC, Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 707] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 152. | Mangano DT, Tudor IC, Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 707] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 153. | Kang Y, Lewis JH, Navalgund A, Russell MW, Bontempo FA, Niren LS, Starzl TE. Epsilon-aminocaproic acid for treatment of fibrinolysis during liver transplantation. Anesthesiology. 1987;66:766-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 123] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 154. | Dalmau A, Sabaté A, Acosta F, Garcia-Huete L, Koo M, Sansano T, Rafecas A, Figueras J, Jaurrieta E, Parrilla P. Tranexamic acid reduces red cell transfusion better than epsilon-aminocaproic acid or placebo in liver transplantation. Anesth Analg. 2000;91:29-34. [PubMed] |
| 155. | Dalmau A, Sabaté A, Acosta F, Garcia-Huete L, Koo M, Reche M, Rafecas A, Figueras J, Jaurrieta E. Comparative study of antifibrinolytic drugs in orthotopic liver transplantation. Transplant Proc. 1999;31:2361-2362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 156. | Dalmau A, Sabaté A, Koo M, Rafecas A, Figueras J, Jaurrieta E. Prophylactic use of tranexamic acid and incidence of arterial thrombosis in liver transplantation. Anesth Analg. 2001;93:516. [PubMed] |
| 157. | Dalmau A, Sabaté A, Koo M, Bartolomé C, Rafecas A, Figueras J, Jaurrieta E. The prophylactic use of tranexamic acid and aprotinin in orthotopic liver transplantation: a comparative study. Liver Transpl. 2004;10:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 158. | Cerutti E, Stratta C, Romagnoli R, Schellino MM, Skurzak S, Rizzetto M, Tamponi G, Salizzoni M. Thromboelastogram monitoring in the perioperative period of hepatectomy for adult living liver donation. Liver Transpl. 2004;10:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 159. | Richards EM, Alexander GJ, Calne RY, Baglin TP. Thrombocytopenia following liver transplantation is associated with platelet consumption and thrombin generation. Br J Haematol. 1997;98:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 160. | Faeh M, Hauser SP, Nydegger UE. Transient thrombopoietin peak after liver transplantation for end-stage liver disease. Br J Haematol. 2001;112:493-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 161. | Goulis J, Chau TN, Jordan S, Mehta AB, Watkinson A, Rolles K, Burroughs AK. Thrombopoietin concentrations are low in patients with cirrhosis and thrombocytopenia and are restored after orthotopic liver transplantation. Gut. 1999;44:754-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 93] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 162. | Sutedja DS, Wai CT, Teoh KF, Lee HL, DaCosta M, Kaur M, Lee YM, Lee KH, Mak K, Quak SH. Persistent thrombocytopenia in liver transplant patients. Transplant Proc. 2004;36:2331-2333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |