Published online Dec 21, 2006. doi: 10.3748/wjg.v12.i47.7613
Revised: November 12, 2006
Accepted: November 20, 2006
Published online: December 21, 2006
AIM: To construct a tumor-selective replication-competent adenovirus (RCAd), SG300, using a modified promoter of human telomerase reverse transcriptase (hTERT).
METHODS: The antitumor efficacy of SG300 in hepatocellular carcinoma was assessed in vitro and
in vivo. In vitro cell viability by MTT assay was used to assess the tumor-selective oncolysis and safety features of SG300, and in vivo antitumor activity of SG300 was assessed in established hepatocellular carcinoma models in nude mice.
RESULTS: SG300 could lyse hepatocellular carcinoma cells at a low multiplicity of infection (MOI), but could not affect growth of normal cells even at a high MOI. Both in Hep3B and SMMC-7721 xenograft models of hepatocellular carcinoma, SG300 had an obvious antitumor effect, resulting in a decrease in tumor volume. Its selective oncolysis to tumor cells and safety to normal cells was also superior to that of ONYX-015. Pathological examination of tumor specimens showed that SG300 replicated selectively in cancer cells and resulted in apoptosis and necrosis of cancer cells.
CONCLUSION: hTERT promoter-regulated replicative adenovirus SG300 has a better cancer-selective replication-competent ability, and can specifically kill a wide range of cancer cells with positive telomerase activity, and thus has better potential for targeting therapy of hepatocellular carcinoma.
- Citation: Su CQ, Wang XH, Chen J, Liu YJ, Wang WG, Li LF, Wu MC, Qian QJ. Antitumor activity of an hTERT promoter-regulated tumor-selective oncolytic adenovirus in human hepatocellular carcinoma. World J Gastroenterol 2006; 12(47): 7613-7620
- URL: https://www.wjgnet.com/1007-9327/full/v12/i47/7613.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i47.7613
Hepatocellular carcinoma (HCC) is one of the malignant diseases with a high incidence and mortality in China. Today, malignant tumors have already become the first death cause in Chinese city residents, and the second in country residents. Among various malignant tumors, HCC is the second cause of cancer death, next only to lung cancer. Based on recent incidence and mortality data available, the International Arctic Research Center (IARC) estimated that there were about 564 000 new cases of HCC in 2000 worldwide, in which 306 000 people were in China. About 300 000 people died of HCC annually in China[1]. The mortality of HCC is more than 40/100 000, especially in the high-risk area of Qidong, Jiangsu Province. HCC severely threatens people’s life partially because of lack of effective therapeutic methods in clinics, and most strategies of gene therapy are not quite satisfactory. Therefore, to develop a therapeutic approach mainly targeting at HCC and synchronously at other tumors is an urgent task.
In cancer gene therapy, human adenoviruses have been used extensively[2], because they can infect most types of human cells, carry a large fragment of foreign sequence, and be grown in cultures to high-titered stable stocks[3]. Most adenoviral vectors used in gene transfer are replication-deficient because the adenoviral E1 region is deleted and replaced by antitumor therapeutic genes[4]. The first generated replication-deficient adenovirus (RDAd) lacks only one (E1) or two (E1 and E3) early genes. The second and third ones also contain E2 and/or E4 deletions[5,6]. Although the clinical protocols employing RDAd have been large in number[6], all of them have major problems. Since RDAd cannot replicate, they express foreign therapeutic genes at low levels only in cells that are initially infected. As a result, the antitumor effect is easily lost rapidly and the bystander effect on proximal cancer cells is limited. Another problem of RDAd is lack of specificity targeting cancer cells[7], which may further decrease the therapeutic effect on cancer cells and result in toxicity to normal cells. Therefore, development of novel effective tumor-targeting adenoviral vectors to enhance the efficiency and specificity of transgene expression is needed.
Recently, cancer-selective replication-competent adenovirus (RCAd) has attracted more attention in the field of cancer gene therapy. RCAds can specifically replicate in cancer cells, induce cell death and release progeny virions. The progeny virions will continue to infect neighboring cancer cells, thus magnifying the oncolytic effect[8]. In this approach of virotherapy, the specificity of the viral agent to selectively kill cancer cells, while relatively spare non-cancer cells, is achieved by controlling viral replication[9]. Abnormal biological features shared by various cancers have been used to restrict replication of adenoviruses to cancer cells. Recently the promoter of human telomerase reverse transcriptase (hTERT) was used to restrict adenoviral replication to telomerase-positive cancer cells through controlling the adenoviral E1a gene or E4 gene[10,11]. The results suggested that the tight regulation of the adenoviral E1a gene is more crucial for replication of viruses. Compared with other promoters, the hTERT promoter is highly active in more than 85% of different human cancers, but inactive in most normal somatic cells, and thus this mechanism can be applied to a wide range of cancers.
We constructed an hTERT promoter-regulated RCAd, designated SG300, in which the adenoviral E1a gene was driven by the hTERT promoter core sequence (-212 bp to +46 bp) with additional 3 E-boxes (CACGTG). It can selectively replicate in a broad array of human cancer cells with positive telomerase activity, but not in normal cells[12]. However, its effect in treating HCC in vitro and in vivo is unknown. In this study, we further tested the antitumor activity of the tumor-selective RCAd, SG300, and confirmed its highly efficient antitumor activity in vitro in several types of cancer cell lines and in vivo in xenograft models of HCC.
Human primary HCC cell lines Hep3B and HepG2, and human normal fibroblast cell lines MRC-5 and IMR-90 were purchased from American Type Culture Collection (ATCC, Manassas, VA). Human HCC cell lines BEL-7401 and SMMC-7721, and human normal hepatocellular line L02 were obtained from the Institute of Cell Biology, Chinese Academy of Sciences, Shanghai, China. All cell lines were cultured in media recommended by the providers: MRC-5, and IMR-90 in modified Eagle’s medium (Gibco BRL, Gaithersburg, MD), and all other cell lines in Dulbecco’s modified Eagle’s medium (GibcoBRL) in a 5% CO2 atmosphere at 37°C. All media were supplemented with 10% heat-inactivated fetal bovine serum (FBS) containing 100 U/mL penicillin and 100 μg/mL streptomycin. Telomerase activity of every cell line was measured by the telomeric repeat amplification protocol (TRAP) and ELISA assay with TRAP-PCR-ELISA telomerase detection kit (Chemicon International, Temecula, CA) according to the protocols.
hTERT promoter core sequence (-212 bp to +46 bp) containing three E-box (CACGTG) motifs was synthesized and cloned into plasmid pMD18-T (TaKaRa Biotech. Co., Ltd., Ohtsu, Japan), and plasmid pCAG166TP was generated. After sequencing, the fragment containing hTERT promoter released from pCAG166TP was inserted into upstream of E1a gene of the adenoviral vector plasmid pXC1 (Microbix Biosystem Inc.), which has type 5 adenovirus sequence from 22 bp to 5790 bp containing E1 gene. The obtained novel adenoviral vector plasmid, named pXC20-TP, was co-transfected with pBHGE3 (Microbix Biosystem Inc.), a plasmid of type 5 adenoviral right arm, into HEK293 cells using the Effectene Transfection Reagent (QIAGEN Inc.) to construct the recombinant adenovirus. About 9 to 14 d after transfection, single white plagues emerged in HEK293 cells. After plague purification three times, we obtained a recombinant replicative adenovirus designated SG300, in which E1a gene expression was driven by the hTERT core promoter. SG300 was amplified in HEK293 cells, extracted using QIAamp DNA Blood Mini Kit (QIAGEN Inc.), and purified by ultra-centrifugation on cesium chloride (CsCl) gradients.
Cell viability was measured by MTT assay using Cell Proliferation Kit I (Roche Molecular Biochemicals, Indianapolis, IN). Serial concentrations of cells were diluted from 2 × 104 to 2 × 105 cells/mL, and seeded in 96-well plates at 100 μL per well. Every concentration was installed in 8 wells. After cultured for 24 h, 100 μL medium per well without FBS was added and cells were continuously cultured for another 7 d. After removal of culture medium from the plates, 100 μL 0.1 mol/L PBS and 10 μL MTT labeling reagent per well were added. The plates were then placed in an incubator for 4 h. After 100 μL solubilization solution was added per well and cultured overnight, the plates were examined with Microplate Reader Model 550 (BIO-RAD Laboratories, Tokyo, Japan) at 570 nm with a reference of 655 nm. Cell viability curves were drawn, on which the most suitable cell concentrations or densities were defined. To test the multiplicity of infection (MOI) of cell viability, every cell line was plated at the density defined above in 96-well plates and infected 24 h later with SG300, ONYX-015 (a control replicative adenovirus, kindly given by Berk AJ, University of California-Los Angeles, Los Angeles, CA), and wild-type adenovirus 5 (WAd5) at a wide range of MOIs from 0.0001 to 500 pfu/cell. Seven days after infection, MTT assays were performed as described above.
Cells were plated in 6-well plates at 106 cells per well and infected with the replicative adenovirus SG300 or ONYX-015 at an MOI of 1 pfu/cell synchronously with MTT assay. At 48 h after infection, the cells and supernatants were harvested, and their viral titers were examined with TCID50 method. The titer data were normalized to that at the beginning of infection and expressed as replicative times.
BALB/c nude mice aged 6 to 8 wk were purchased from Shanghai Experimental Animal Center, Chinese Academy of Sciences, Shanghai, China. Human HCC cell lines, SMMC-7721 and Hep3B, in log phase (107 cells in 100 μL medium) were subcutaneously injected into the right flanks of mice. When tumors were induced, mice were divided randomly into four groups as SMMC-7721 models (SG300, ONYX-015, WAd5 and control groups, n = 10 mice/group), and three groups as Hep3B models (SG300, ONYX-015 and control groups, n = 10 mice/group), and given intratumoral injection of 2 × 108 pfu viruses in a volume of 100 μL viral preservation solution (10 mmol/L Tris-HCl pH 8.0, 2 mmol/L MgCl2, 4% sucrose), once every other day with total injections of five times and total dosage of 109 pfu per mouse in the SG300, ONYX-015 and WAd5 groups, respectively. In the control group, mice received injections of the same volume of viral preservation solution five times. Tumor sizes were measured regularly using calipers. Tumor volumes were estimated with the following formula: a×b2× 0.5, in which a and b represent the maximal and minimal diameters, respectively.
Mice were killed on d 42 after treatment in SMMC-7721 model group and on d 56 in Hep3B model group by cervical dislocation. Specimens from the tumor, liver, and lung were collected, fixed in 10% neutral formaldehyde for 6 h and paraffin-embedded, and 5 μm-thick consecutive sections were sliced for pathological examination. The expression of adenoviral capsid protein, hexon, was located using mouse anti-adenoviral hexon antibody (Biodesign International, MA, USA). To demonstrate apoptotic cell death of tumor tissues on paraffin-embedded sections, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay was performed using In Situ Cell Death Detection Kit (Roche Diagnostics, Basel, Switzerland) according to the manufacturer’s instructions. Positive index (PI) was counted from five randomly selected high-power fields under light microscope, and expressed as a percentage of total cells counted.
Experiments were performed three times and the data were presented as mean ± SD. Student’s t-test was carried out to assess the statistical difference. P < 0.05 was considered to be significant.
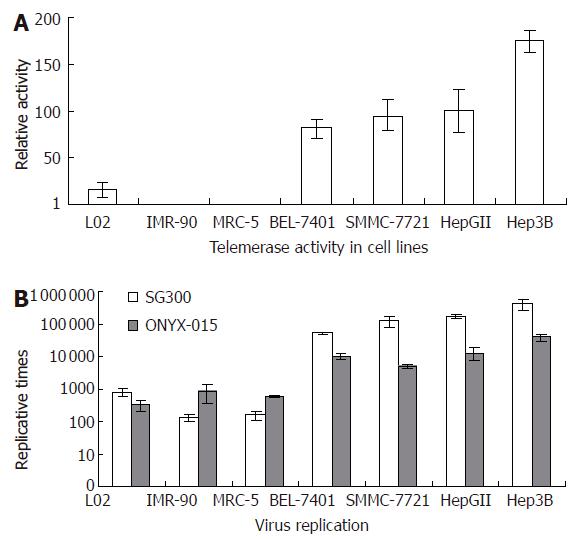
By TRAP-ELISA assay, telomerase activity of cultured cell lines was measured. It showed that all cancer cell lines were positive for telomerase activity; however, the two normal fibroblast cell lines were not. The normal hepatocellular line L02 showed weak telomerase activity (Figure 1A).
By TCID50 method, we measured virus titers on d 4 after infected with SG300 and ONYX-015, respectively. The replicative capability of SG300 was markedly enhanced in different cancer cell lines with increases of virus yields by 50 793 to 406 250 times. In normal fibroblast cell lines, however, SG300 had a lower replicative capability. Its replicative times were below 160 (P = 0.0453). It showed a certain replicative activity in human hepatocellular cell line L02, with replicative times of 796 (Figure 1B). ONYX-015 replicated more slowly than SG300 in cancer cells (P = 0.0348).
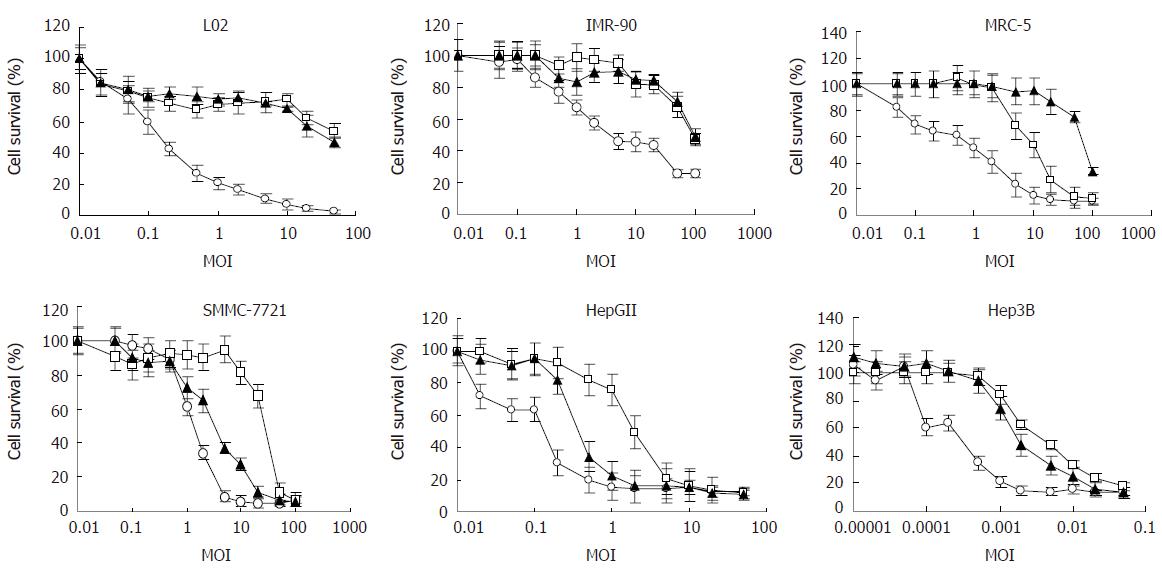
To quantify the viability of cells, MTT assay was used. First, a suitable cell concentration or density for each cell line was determined, at which cells were seeded in 96-well plates and would not induce any cytopathic effect (CPE) within 7 d. This was to make sure that the killing effect that might appear in the following experiments was due to adenoviral replication. Second, we investigated the viability of cells, including HCC cell lines and normal cell lines, respectively infected with SG300, ONYX-015 and WAd5, which reflected the selectivity and safety of SG300. The results showed that SG300 could lyse HCC cells at a very low MOI (Figure 2). Seven days after infection of SG300 at an MOI of 10 pfu/cell, the viability of each HCC cell line was below 30% (15.3%, 27.6% in HepGII, SMMC-7721, respectively); however, at the same MOI, the viability of each normal cell line was higher (68.0%, 84.8%, 94.0% in L02, IMR-90, MRC-5, respectively). The IC50s (MOI values of 50% viability) of SG300 in cancer cells were markedly lower than that in normal cells (P = 0.0111) (Table 1). Hep3B was very sensitive to SG300, and cells were killed obviously and cell viability decreased to 13.2% at an MOI of 0.05 pfu/cell. ONYX-015 also had a killing effect on cancer cells, but it required a higher MOI than SG300 to reach the same effective level as SG300. The viability of HepGII or SMMC-7721 infected with ONYX-015 was 16.3% or 81.1% at an MOI of 10 pfu/cell, and of Hep3B was 17.8% at an MOI of 0.05 pfu/cell. There was no difference between the IC50s of WAd5 in cancer cells and normal cells (P = 0.4081). MTT assay directly showed that the killing effect of SG300 was several times or even more than that of ONYX-015 on HCC cells, but weaker than that of ONYX-015 on normal cells.
| Cells | SG300 | ONYX-015 | WAd5 |
| L02 | 41.5658 ± 2.2122 | 58.0351 ± 8.9114 | 0.1520 ± 0.0099 |
| IMR-90 | 94.5783 ± 5.2813 | 90.4534 ± 8.2359 | 2.1342 ± 0.0897 |
| MRC-5 | 82.5674 ± 8.5549 | 14.2622 ± 1.7781 | 1.1525 ± 0.0336 |
| SMMC-7721 | 2.8954 ± 0.0641a | 25.5323 ± 6.2351 | 1.3426 ± 0.0625 |
| HepGII | 0.3232 ± 0.0085a | 1.9845 ± 0.0046a | 0.1123 ± 0.0030 |
| Hep3B | 0.0017 ± 0.0003a | 0.0042 ± 0.0005a | 0.0013 ± 0.0002 |
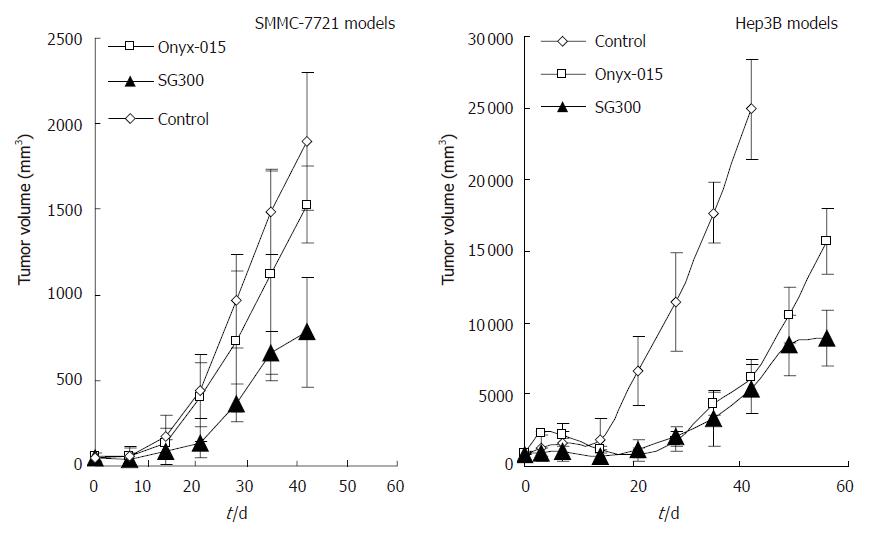
Hep3B and SMMC-7721 cells were implanted into BALB/c nude mice to induce subcutaneous tumors. Tumors were seen 20 d later after implantation of SMMC-7721 cells, and about 30 d later after implantation of Hep3B cells. The adenoviruses and the control buffer (viral preservation solution) were injected intralesionally. Forty-two days later, half mice (5/10) died in WAd5 group of SMMC-7721 models. The groups injected with adenoviruses both in these two models of HCC showed antitumor effects to some extent when compared with the control group (P = 0.0001, 0.0364 for SG300 and ONYX-015, respectively, in SMMC-7721 models; P = 0.0005, 0.0019 for SG300 and ONYX-015, respectively, in Hep3B models), and the effects of SG300, being close to that of WAd5, were better than that of ONYX-015 (P = 0.0029, 0.0066 in SMMC-7721 and Hep3B models, respectively) (Figure 3). Mice of the control group in Hep3B models were sacrificed ahead of time on d 42 after treatment by cervical dislocation due to overloading of tumors.
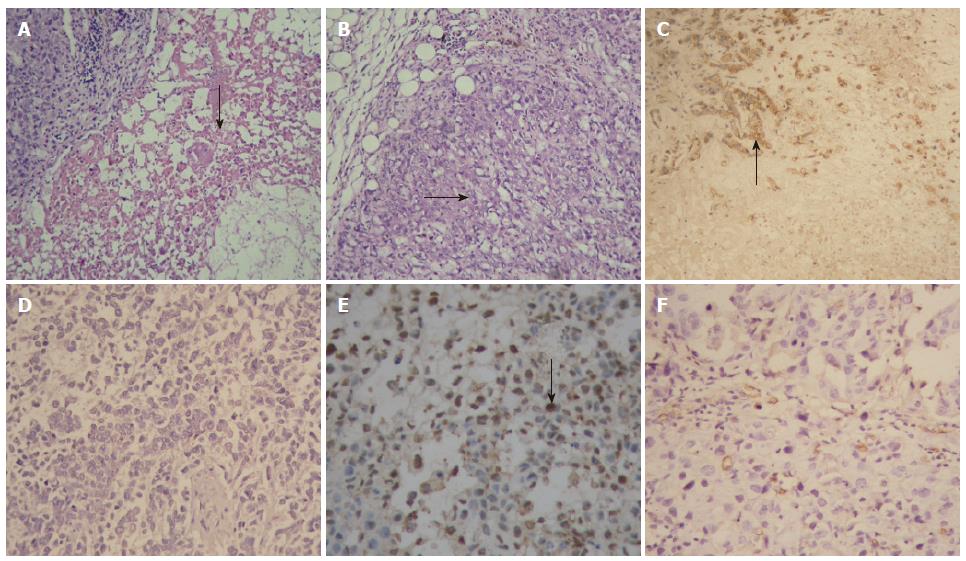
Pathological examination showed that there were many areas of necrosis in tumor tissues of the SG300-treated group and ONYX-015-treated group. In the control group, however, cancer cells grew luxuriantly with only a few, small focal areas of necrosis (Figure 4A and B). Immunohistochemistry demonstrated that most cancer cells positive for hexon were distributed mainly around the necrotic areas (Figure 4C and D), and that more apoptotic cancer cells were positive for TUNEL labeling in tumor tissues of the SG300-treated group, compared with the control group (Figure 4E and F). PIs in SG300, ONYX-015 and control groups were 59.4 ± 21.1, 42.3 ± 18.2, 17.5 ± 8.9 for TUNEL labeling, respectively. There was a difference between TUNEL labeling PI in the control group and in any other replicative adenovirus-treated group (P = 0.0007 for SG300, P = 0.0032 for ONYX-015). The liver and lung tissues of mice were negative for hexon in SG300-treated group as well as ONYX-015 and control groups.
Virotherapy employing tumor-selective replication-competent adenovirus (RCAd) is a promising strategy in cancer treatment. An alternative approach to restrict adenoviral replication to targeting cancer cells and achieve RCAd is to modify adenovirus by partial deletion of viral genes that are essential for replication in normal cells but not in tumor cells[13-15]. The representative RCAd of this type is ONYX-015[16]. It has a deletion of E1b-55K gene and replicates specifically in cancer cells lacking wide-type p53[17,18]. An additional approach to achieve RCAd is to place viral replicative genes under the control of cancer-specific promoters that are activated only in cancer cells[19]. Several cancer-specific promoters that are more active in particular cancer cells but inactive or only weakly active in cancer-originating somatic cells, have been identified and explored to construct RCAds, such as the carcinoembryonic antigen (CEA) promoter targeting colorectal and lung cancer[20], the α-fetoprotein promoter targeting hepatocellular cancer[21,22], the prostate-specific antigen (PSA) promoter targeting prostate cancer[2], the MUC1 antigen promoter targeting breast cancer[7], the E2F transcriptional factor promoter targeting cancers with a defective pRb/E2F/p16 pathway[23-25], the surfactant protein B (SPB) promoter targeting lung cancer[3], and the L-plastin promoter targeting ovarian and bladder cancers[26]. These cancer-specific promoters can provide RCAds, the selective replication in corresponding cancer cells and demonstrate antitumor activity in preclinical models and clinical trials. However, most of them are limited, targeting a narrow range of cancers expressing the corresponding tumor antigen, thus attenuating their efficacy in cancer therapy[27].
A number of gene therapeutic approaches have been proposed to kill cancer cells or inhibit growth of caners by targeting telomerase[28]. Most of them utilize the hTERT promoter to drive antitumor gene expression selectively in cancer cells with positive telomerase activity, without affecting normal cells negative for telomerase[29,30]. It indicates the potential therapeutic application of the strategy targeting telomerase for a wide range of various cancers. Recently the hTERT promoter has been tested for targeting cancer gene therapy with replication-competent viral vectors. It was used to control the adenoviral early gene expression and produce a series of RCAds, but experimental results were inconsistent. Some studies showed that the hTERT promoter provided a sufficient selection for viral replication in cancer cells and did not in normal cells when it was used to construct CRAds by controlling E1a gene[10,31,32]. Other studies demonstrated that the hTERT promoter failed to block viral replication in telomerase-negative cells when it was used to control E4 gene expression[11]. Because the wild-type hTERT promoter is incapable of mediating a high level of RCAd replication in cancer cells, it is necessary to develop a modified form of the promoter with improved transcriptional activity and cancer specificity. Wirth and coworkers reported that the transcriptional activity of the hTERT promoter could be enhanced significantly in a majority of tumor cell lines by inserting the E1a TATA box upstream of the transcription start site of its controlled gene[33]. This modification in an RCAd, named hTERT-Ad, did not alter the tumor-selective specificity of the promoter. Kim et al[34] constructed a modified hTERT promoter-controlled RCAd, Ad-mTERT-Δ19, in which a fragment containing one copy of c-Myc binding site and five copies of SP1 binding sites was incorporated into the downstream region of the wild-type hTERT promoter. The experimental results strongly suggested that the transcriptional activity of the modified hTERT promoter was up-regulated in cancer cells but not in normal cells, and the replicative capability and cytolytic effect of Ad-mTERT-Δ19 were enhanced only in cancer cells compared with wild-type hTERT promoter-controlled RCAd, Ad-TERT-Δ19. To further increase the tumor-selective specificity of RCAd controlled by the hTERT promoter and decrease the adenoviral toxicity to normal cells, we previously used a modified hTERT promoter with three additional copies of E-boxes (CACGTG) to construct an RCAd, named SG300. The results suggested that by this modification, the specificity of RCAd targeting cancer cells could be improved and its toxicity to normal cells could be reduced[12].
Our experiments in vitro showed that SG300 lysed or destroyed cancer cells at a low MOI, including human HCC cell lines, Hep3B, HepGII, BEL-7401 and SMMC-7721, but did not affect growth of normal cells such as human normal fibroblast cell lines, MRC-5 and IMR-90, even at relative high MOIs. The oncolytic ability of CRAds varied in different types of HCC cells. Both SG300 and ONYX-015 had strong effects on Hep3B. The selective cytolytic ability of SG300 was more powerful than that of ONYX-015 in HCC cells. The effect of SG300 on normal cells was less than that of ONYX-015. ONYX-015 is an oncolytic adenovirus that shows different mechanisms and replicates preferably in p53-deficient tumor cells. Because the positive rate of telomerase activity was higher than the incidence rate of p53-deficiency in human cancers, ONYX-015 targets a narrower range of cancer types than SG300. Furthermore, our previous studies and the current data proved that the replicative capacity of ONYX-015 in tumor cells was weaker than that of SG300 series[12]. Probably that is why the selective cytolytic ability of ONYX-015 was weaker than that of SG300 in HCC cells. All these results, combined with the data of telomerase activity and viral replicative capability in cultured cell lines, demonstrated that SG300 has a better cancer-selective replication-competent ability, and can specifically kill a wide range of cancer cells with positive telomerase activity. The killing effect of SG300 was not only consistent with its replicative capacity in different cell lines but also with the telomerase activity of these cells. L02 cells showed a weak telomerase activity, and SG300 could replicate in this cell line to some extent and resulted in weak cytolysis.
BALB/c nude mice were subcutaneously injected with Hep3B and SMMC-7721 cells, respectively. When the tumors were induced, the replicative adenoviruses were injected intralesionally with a total dosage of 109 pfu per mouse. The results showed that SG300 yielded an obvious antitumor effect on HCC xenografts. Tumor growth was inhibited with a decrease in tumor volume. The antitumor effect of SG300 was better than that of ONYX-015. During the period of observation, half mice of WAd5-infected group in SMMC-7721 models died of virus toxicity, but no mice died in SG300-treated groups either in SMMC-7721 or in Hep3B models, demonstrating that SG300 had no toxicity to normal tissues or organs and therefore, was safe in cancer therapy. Pathological examinations showed that SG300 replicated selectively in HCC cells and resulted in apoptosis and necrosis of cancer cells. In brief, hTERT promoter-regulated replicative adenovirus SG300 has a selective replicative ability and a specific cytolytic ability targeting HCC cells and other cancer cells that are positive for telomerase activity. It shows superior selective replication and antitumor effect when compared with ONYX-015, and thus has better potential for HCC therapy.
We thank Lihua Jiang and Yanzhen Qian of our laboratory for their assistance with cell culture; Jianzhong Gu, of Shanghai Experimental Animal Center, Chinese Academy of Sciences, for his help with animal studies. We also thank Berk AJ, University of California-Los Angeles, Los Angeles, for kindly providing the control replicative adenovirus ONYX-015.
Recently, a number of gene therapeutic approaches by cancer-selective replication-competent adenovirus (RCAd) have been proposed in the field of cancer gene therapy, and attracted much attention. However, in vitro experimental results are inconsistent. The objective of this study was to further increase the tumor-selective specificity of RCAd and decrease the adenoviral toxicity to normal cells.
In this study we constructed SG300 by use of a modified hTERT promoter. It could lyse hepatocellular carcinoma cells at a low multiplicity of infection (MOI), but did not affect growth of normal cells even at a high MOI. Its selective oncolytic ability was stronger than that of ONYX-015, which is the representative selective replicative adenovirus, and its influence on normal cells was less than that of ONYX-015.
To further increase the tumor-selective specificity of RCAd controlled by the hTERT promoter and decrease the adenoviral toxicity to normal cells, we constructed an RCAd, SG300, by use of a modified hTERT promoter with three additional copies of E-box (CACGTG). Its antitumor activity in vitro and in vivo was shown to be excellent. Both in Hep3B and SMMC-7721 xenograft models of hepatocellular carcinomas, SG300 had an obvious antitumor effect, resulting in a decrease in tumor volume due to its selective replication in cancer cells and its specific oncolysis.
hTERT promoter-regulated replicative adenovirus SG300 has a selective replicative ability and a specific cytolytic ability targeting hepatocellular carcinoma cells and other cancer cells that are positive for telomerase activity. It displays superior selective replication and antitumor effect when compared with ONYX-015, and thus has better potential for HCC therapy.
RCAd: replication-competent adenovirus; hTERT: human telomerase reverse transcriptase
hTERT promoter-regulated replicative adenovirus SG300 has a better cancer-selective replication-competent ability, and can specifically kill a wide range of cancer cells with positive telomerase activity, and thus has better potential for targeting therapy of hepatocellular carcinoma.
S- Editor Liu Y L- Editor Zhu LH E- Editor Ma WH
| 1. | Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37 Suppl 8:S4-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1370] [Cited by in RCA: 1370] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 2. | Rodriguez R, Schuur ER, Lim HY, Henderson GA, Simons JW, Henderson DR. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Res. 1997;57:2559-2563. [PubMed] |
| 3. | Doronin K, Kuppuswamy M, Toth K, Tollefson AE, Krajcsi P, Krougliak V, Wold WS. Tissue-specific, tumor-selective, replication-competent adenovirus vector for cancer gene therapy. J Virol. 2001;75:3314-3324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 88] [Reference Citation Analysis (0)] |
| 4. | Yang C, Cirielli C, Capogrossi MC, Passaniti A. Adenovirus-mediated wild-type p53 expression induces apoptosis and suppresses tumorigenesis of prostatic tumor cells. Cancer Res. 1995;55:4210-4213. [PubMed] |
| 5. | Andrews JL, Kadan MJ, Gorziglia MI, Kaleko M, Connelly S. Generation and characterization of E1/E2a/E3/E4-deficient adenoviral vectors encoding human factor VIII. Mol Ther. 2001;3:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1851] [Cited by in RCA: 1838] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 7. | Kurihara T, Brough DE, Kovesdi I, Kufe DW. Selectivity of a replication-competent adenovirus for human breast carcinoma cells expressing the MUC1 antigen. J Clin Invest. 2000;106:763-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 171] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Kirn D, Martuza RL, Zwiebel J. Replication-selective virotherapy for cancer: Biological principles, risk management and future directions. Nat Med. 2001;7:781-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 377] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 9. | Curiel DT. The development of conditionally replicative adenoviruses for cancer therapy. Clin Cancer Res. 2000;6:3395-3399. [PubMed] |
| 10. | Huang TG, Savontaus MJ, Shinozaki K, Sauter BV, Woo SL. Telomerase-dependent oncolytic adenovirus for cancer treatment. Gene Ther. 2003;10:1241-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Hernandez-Alcoceba R, Pihalja M, Qian D, Clarke MF. New oncolytic adenoviruses with hypoxia- and estrogen receptor-regulated replication. Hum Gene Ther. 2002;13:1737-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Su CQ, Sham J, Xue HB, Wang XH, Chua D, Cui ZF, Peng LH, Li LF, Jiang LH, Wu MC. Potent antitumoral efficacy of a novel replicative adenovirus CNHK300 targeting telomerase-positive cancer cells. J Cancer Res Clin Oncol. 2004;130:591-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Li G, Sham J, Yang J, Su C, Xue H, Chua D, Sun L, Zhang Q, Cui Z, Wu M. Potent antitumor efficacy of an E1B 55kDa-deficient adenovirus carrying murine endostatin in hepatocellular carcinoma. Int J Cancer. 2005;113:640-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Biederer C, Ries S, Brandts CH, McCormick F. Replication-selective viruses for cancer therapy. J Mol Med (Berl). 2002;80:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Ring CJ. Cytolytic viruses as potential anti-cancer agents. J Gen Virol. 2002;83:491-502. [PubMed] |
| 16. | Ries S, Korn WM. ONYX-015: mechanisms of action and clinical potential of a replication-selective adenovirus. Br J Cancer. 2002;86:5-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Morley S, MacDonald G, Kirn D, Kaye S, Brown R, Soutar D. The dl1520 virus is found preferentially in tumor tissue after direct intratumoral injection in oral carcinoma. Clin Cancer Res. 2004;10:4357-4362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Hann B, Balmain A. Replication of an E1B 55-kilodalton protein-deficient adenovirus (ONYX-015) is restored by gain-of-function rather than loss-of-function p53 mutants. J Virol. 2003;77:11588-11595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Saukkonen K, Hemminki A. Tissue-specific promoters for cancer gene therapy. Expert Opin Biol Ther. 2004;4:683-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Li Y, Chen Y, Dilley J, Arroyo T, Ko D, Working P, Yu DC. Carcinoembryonic antigen-producing cell-specific oncolytic adenovirus, OV798, for colorectal cancer therapy. Mol Cancer Ther. 2003;2:1003-1009. [PubMed] |
| 21. | Kim J, Lee B, Kim JS, Yun CO, Kim JH, Lee YJ, Joo CH, Lee H. Antitumoral effects of recombinant adenovirus YKL-1001, conditionally replicating in alpha-fetoprotein-producing human liver cancer cells. Cancer Lett. 2002;180:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Li Y, Yu DC, Chen Y, Amin P, Zhang H, Nguyen N, Henderson DR. A hepatocellular carcinoma-specific adenovirus variant, CV890, eliminates distant human liver tumors in combination with doxorubicin. Cancer Res. 2001;61:6428-6436. [PubMed] |
| 23. | Ryan PC, Jakubczak JL, Stewart DA, Hawkins LK, Cheng C, Clarke LM, Ganesh S, Hay C, Huang Y, Kaloss M. Antitumor efficacy and tumor-selective replication with a single intravenous injection of OAS403, an oncolytic adenovirus dependent on two prevalent alterations in human cancer. Cancer Gene Ther. 2004;11:555-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Johnson L, Shen A, Boyle L, Kunich J, Pandey K, Lemmon M, Hermiston T, Giedlin M, McCormick F, Fattaey A. Selectively replicating adenoviruses targeting deregulated E2F activity are potent, systemic antitumor agents. Cancer Cell. 2002;1:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Tsukuda K, Wiewrodt R, Molnar-Kimber K, Jovanovic VP, Amin KM. An E2F-responsive replication-selective adenovirus targeted to the defective cell cycle in cancer cells: potent antitumoral efficacy but no toxicity to normal cell. Cancer Res. 2002;62:3438-3447. [PubMed] |
| 26. | Akbulut H, Zhang L, Tang Y, Deisseroth A. Cytotoxic effect of replication-competent adenoviral vectors carrying L-plastin promoter regulated E1A and cytosine deaminase genes in cancers of the breast, ovary and colon. Cancer Gene Ther. 2003;10:388-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Gu J, Andreeff M, Roth JA, Fang B. hTERT promoter induces tumor-specific Bax gene expression and cell killing in syngenic mouse tumor model and prevents systemic toxicity. Gene Ther. 2002;9:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Hodes R. Molecular targeting of cancer: telomeres as targets. Proc Natl Acad Sci USA. 2001;98:7649-7651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Koga S, Hirohata S, Kondo Y, Komata T, Takakura M, Inoue M, Kyo S, Kondo S. A novel telomerase-specific gene therapy: gene transfer of caspase-8 utilizing the human telomerase catalytic subunit gene promoter. Hum Gene Ther. 2000;11:1397-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Gu J, Fang B. Telomerase promoter-driven cancer gene therapy. Cancer Biol Ther. 2003;2:S64-S70. [PubMed] |
| 31. | Kawashima T, Kagawa S, Kobayashi N, Shirakiya Y, Umeoka T, Teraishi F, Taki M, Kyo S, Tanaka N, Fujiwara T. Telomerase-specific replication-selective virotherapy for human cancer. Clin Cancer Res. 2004;10:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 179] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 32. | Irving J, Wang Z, Powell S, O'Sullivan C, Mok M, Murphy B, Cardoza L, Lebkowski JS, Majumdar AS. Conditionally replicative adenovirus driven by the human telomerase promoter provides broad-spectrum antitumor activity without liver toxicity. Cancer Gene Ther. 2004;11:174-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Wirth T, Zender L, Schulte B, Mundt B, Plentz R, Rudolph KL, Manns M, Kubicka S, Kühnel F. A telomerase-dependent conditionally replicating adenovirus for selective treatment of cancer. Cancer Res. 2003;63:3181-3188. [PubMed] |
| 34. | Kim E, Kim JH, Shin HY, Lee H, Yang JM, Kim J, Sohn JH, Kim H, Yun CO. Ad-mTERT-delta19, a conditional replication-competent adenovirus driven by the human telomerase promoter, selectively replicates in and elicits cytopathic effect in a cancer cell-specific manner. Hum Gene Ther. 2003;14:1415-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |