Published online Dec 7, 2006. doi: 10.3748/wjg.v12.i45.7365
Revised: August 28, 2006
Accepted: September 11, 2006
Published online: December 7, 2006
AIM: To rapidly quantify hepatitis B virus (HBV) DNA by real-time PCR using efficient TaqMan probe and extraction methods of virus DNA.
METHODS: Three standards were prepared by cloning PCR products which targeted S, C and X region of HBV genome into pGEM-T vector respectively. A pair of primers and matched TaqMan probe were selected by comparing the copy number and the Ct values of HBV serum samples derived from the three different standard curves using certain serum DNA. Then the efficiency of six HBV DNA extraction methods including guanidinium isothiocyanate, proteinase K, NaI, NaOH lysis, alkaline lysis and simple boiling was analyzed in sample A, B and C by real-time PCR. Meanwhile, 8 clinical HBV serum samples were quantified.
RESULTS: The copy number of the same HBV serum sample originated from the standard curve of S, C and X regions was 5.7 × 104/mL, 6.3 × 102/mL and 1.6 × 103/mL respectively. The relative Ct value was 26.6, 31.8 and 29.5 respectively. Therefore, primers and matched probe from S region were chosen for further optimization of six extraction methods. The copy number of HBV serum samples A, B and C was 3.49 × 109/mL, 2.08 × 106/mL and 4.40 × 107/mL respectively, the relative Ct value was 19.9, 30 and 26.2 in the method of NaOH lysis, which was the efficientest among six methods. Simple boiling showed a slightly lower efficiency than NaOH lysis. Guanidinium isothiocyanate, proteinase K and NaI displayed that the copy number of HBV serum sample A, B and C was around 105/mL, meanwhile the Ct value was about 30. Alkaline failed to quantify the copy number of three HBV serum samples. Standard deviation (SD) and coefficient variation (CV) were very low in all 8 clinical HBV serum samples, showing that quantification of HBV DNA in triplicate was reliable and accurate.
CONCLUSION: Real-time PCR based on optimized primers and TaqMan probe from S region in combination with NaOH lysis is a simple, rapid and accurate method for quantification of HBV serum DNA.
- Citation: Lu YQ, Han JX, Qi P, Xu W, Zu YH, Zhu B. Rapid quantification of hepatitis B virus DNA by real-time PCR using efficient TaqMan probe and extraction of virus DNA. World J Gastroenterol 2006; 12(45): 7365-7370
- URL: https://www.wjgnet.com/1007-9327/full/v12/i45/7365.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i45.7365
Hepatitis B virus (HBV) is a human hepadnavirus that causes acute and chronic hepatitis and hepatocellular carcinoma[1]. Although an effective vaccine has been available for two decades, an estimated 350 million people worldwide are chronically infected with HBV[2]. The conventional ways available for curing this disease are not very efficient. A significant proportion of chronic infections terminate in hepatocellular carcinoma, leading to more than one million deaths annually[3]. The diagnosis and clinical monitoring of HBV infection are based on the detection of viral antigens, antibodies to viral proteins, and circulating viral genome (HBV DNA)[4-6]. There is increasing evidence that measuring the level of HBV DNA in serum is useful in monitoring the efficacy of antiviral therapy, detecting the occurrence of drug-resistant mutants and relapse after discontinuing antiviral therapy[7-9]. An accurate quantitative assay for serum HBV DNA may monitor residual viral load during treatment and allow the timely detection of flares of viral replication that accompany the emergence of variants.
In China, enzyme-linked immunosorbent assay (ELISA) is still a main detection method for HBV infection, but ELISA results neither efficiently reflect serum viral load or hepatitis activity nor monitor the efficacy of antiviral treatments. In recent years, real-time PCR detection assays based on SYBR Green I[10,11] and TaqMan probe[12-19] have been proposed for quantification of HBV DNA in serum. In the former assay, SYBR Green I can specifically bind to double-stranded DNA (dsDNA) rather than to single-stranded DNA (ssDNA). Fluorescence is monitored once each cycle after product extension and increases above background fluorescence at a cycle number that is dependent on the initial template concentration. Unfortunately, the dye detects all dsDNAs, including primer dimer and other nonspecific products.
The real-time PCR method based on the TaqMan probe uses a dual-labeled fluorescent probe containing a reporter dye that is quenched by a second fluorescent dye[20]. Hydrolysis of the probe by polymerase exonuclease activity separates the reporter from the quencher as the amplification proceeds, the fluorescence signal from the reporter increases cumulatively. The cycle at which fluorescence exceeds background, known as the threshold cycle, is inversely related to the initial copy number, thus allowing quantitative analysis.
The efficacy and accuracy of real-time PCR largely depend on the primers and probe[14,21], and are also related to the extraction method of HBV DNA[22,23]. A simple, rapid, efficient method for DNA extraction is crucial to the success of real-time PCR and the subsequent analysis. We describe here a rapid, convenient real-time quantitative assay for serum HBV DNA which combines optimized primers and probe with simple HBV DNA extraction method. The assay was designed to enable accurate quantification of clinical serum samples, which makes it a useful clinical test to monitor serially the efficacy of antiviral therapy.
Serum HBV DNA was extracted by NaOH lysis as previously described[26]. In brief, 50 μL of HBV positive serum was mixed with an equal volume of 0.4 mol/L NaOH and then the mixture was incubated at 80°C for 10 min, followed by centrifugation for 30 s at 15 000 ×g. After that the supernatant was carefully transferred to a new microcentrifuge tube and supplemented with 25 μL of 0.4 mol/L Tris-HCl (pH7.5). Two microliters of HBV DNA was used as a template in PCR.
Three pairs of primers were designed by software Beacon Designer 2.1 in the conserved region of S, C and X gene respectively and synthesized by Sangon Co., Ltd, Shanghai. The sequences of each pair of primers were as follows: RSU (5’-AGAATCCTCACAATACCGCAGAGT-3’) and RSL (5’-CACACGGTAGTTCCCCCTAGAA-3’), RCU (5’-GTCTTTCGGAGTGTGGATTCG-3’) and RCL (5’-CGGCGATTGAGACCTTCGT-3’), RXU (5’-ACTCCCCGTCTGTGCCTTCT-3’) and RXL (5’-CATTCGGTGGGCGTTCAC-3’).
Three amplified products were cloned into the pGEM-T vector (Promega, USA), and then the recombinant HBV plasmids pGEM-S, pGEM-C and pGEM-X were serially diluted from 107 to 103 copies/μL after identification by PCR. One microliter of each diluted recombinant plasmid was used as a standard PCR template.
Taqman probes targeting S, C and X genes were designed by software Beacon Designer 2.1 and synthesized in Sangon Co., Ltd, Shanghai. The sequences of PS, PC, and PX were 5’FAM-AGACTCGTGGTGGACTTCTCTCAAT-TAMARA3’, 5’FAM-TCCCCTAGAAGAAGAACTCCCTCGCCTC-TAMARA3’, and 5’FAM-CCGGACCGTGTGCACTTCGCTT-TAMARA3’, respectively.
Six methods (including guanidinium isothiocyanate, proteinase K, NaI, NaOH lysis, alkaline lysis, as well as boiling) were used to extract serum HBV DNA from three serum samples[24-28]. Two microliters of HBV DNA was used as a template in real-time PCR.
In the methods of GuSCN, proteinase K and NaI lysis, protocol after the lysis process was the same, though components of lysis were different. GuSCN lysate consisted of 1 mol/L Tris-HCl (pH8.0), 0.5 mol/L EDTA (pH8.0), 100 g/L SDS, 200 mg/L proteinase K[24]. Proteinase K lysate contained 10 mmol/L Tris-HCl (pH8.0), 10 mmol/L EDTA (pH8.0), 0.5% SDS, 150 mmol/L NaCl and 200 mg/L proteinase K[27]. NaI extraction buffer included 6 mol/L NaI, 0.5% SDS, 26 mmol/L Tris-HCl (pH8.0) and 13 mmol/L EDTA (pH8.0)[25]. First, 100 μL lysate was added into an equal volume serum, then the mixture was incubated at 37°C for 1 h, 55°C for 2 h and 60°C for 15 min in GuSCN, proteinase K and NaI lysis methods respectively. After that, 200 μL chloroform/isoamyl alcohol (24:1) was added to extract products and the supernatants were removed into new eppendorf tubes after centrifugation at 15 000 ×g for 15 min, followed by alcohol precipitation and further washing[24,25,27]. At last, naturally dried DNA precipitates were dissolved in 30 μL 0.1 × TE.
The easily manipulated alkaline lysis and boiling extraction were similar to the above NaOH lysis, except for the lysate. The lysate used in alkaline lysis was composed of 1 mol/L NaOH, 2 mol/L NaCl and 0.5% SDS[26], while the boiling extraction consisted of an equal volume of PBS[28].
Real-time PCR amplification was performed in 20 μL reaction mixture containing 1 μL standard DNA or 2 μL isolated serum HBV DNA, 2 × PCR reaction buffer [100 mmol/L Tris-HCl (pH8.3), 100 mmol/L KCl, 7.0 mmol/L MgCl2, 400 μmol/L each of the deoxynucleotide triphosphates (dNTP), 1U hot star DNA polymerase Takara, Japan], 5pmol of each pair of primers and 2.5 pmol of corresponding TaqMan probe. All amplification reactions were performed in triplicate. After preparation of the reaction mixtures in 96-well plates, amplification was performed as follows: initial denaturation at 94°C for 3 min, followed by 40 cycles at 94°C for 20 s, 60°C for 40 s. Fluorescence readings were recorded at 60°C in each cycle. Results were analyzed by the software iCyclerTMiQ 3.0a provided with the iCycler system (Bio-Rad, USA).
To evaluate HBV DNA extraction methods, RSU, RSL primers and PS probe were used in real-time PCR. Amplification and analysis were performed as above.
NaOH lysis was performed to extract serum HBV DNA from 8 clinical serum samples. The selected RSU, RSL and PS were used in real-time PCR. The procedures of real-time PCR and standard curve analysis were described as above.
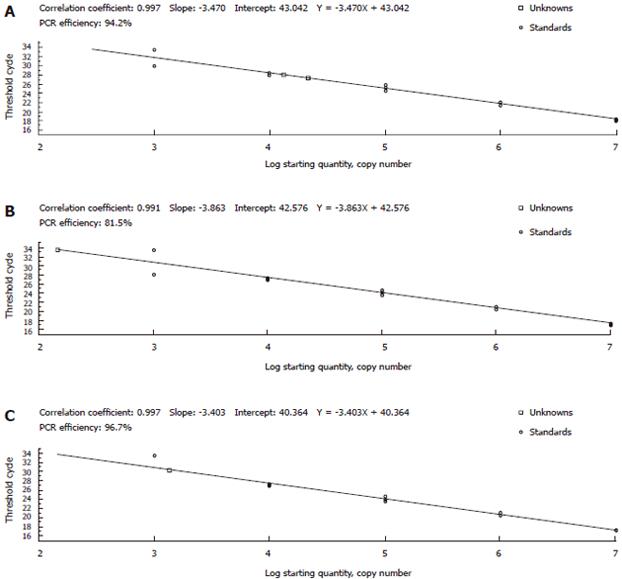
Three recombinant plasmids pGEM-S, pGEM-C and pGEM-X were used as standards to set up the real-time protocol and construct the standard curve. Forty cycles of amplification allowed us to obtain linear quantification between 1 × 103 and 1 × 107 copies/μL per reaction (Figure 1). The highest copy number (5.7 × 104/mL) and the lowest Ct value (26.6) of the same HBV serum samples were observed from the standard curve originated from S region (Table 1). The lowest copy number (6.3 × 102/mL) and the highest Ct value (31.8) were displayed from the standard curve of C region. The highest copy number from S region was about 90.5 and 35.6 multiple to the C and X regions. Hence, RSU, RSL primers and PS probe were chosen for further analysis because of the efficient amplification.
| Standardcurve | Ct | Copy number ( /mL) | ||
| Mean ± SD | CV(%) | Mean ± SD | CV (%) | |
| S | 26.6 ± 0.061 | 0.2 | 5.7 × 104± 2.29 × 103 | 4.0 |
| C | 31.8 ± 0.073 | 0.2 | 6.3 × 102± 2.73 × 101 | 4.3 |
| X | 29.5 ± 0.357 | 1.2 | 1.6 × 103± 1.77 × 102 | 11.1 |
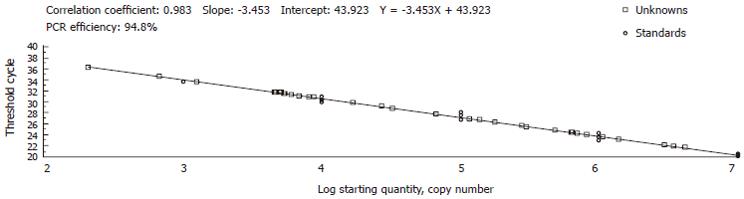
To explore a simple and fast method for serum HBV DNA extraction, six different methods were compared by real-time PCR in combination with selected primers and TaqMan probe from S region. The copy number and Ct values of three HBV serum samples on the standard curve (Figure 2 and Table 2) showed that NaOH lysis and boiling were predominately advantageous over the other methods. Adversely, alkaline lysis failed to detect any HBV DNA in all samples. There was no significant difference among the other three methods.
| Method | Copy number/mL and Ct value | ||
| Sample A | Sample B | Sample C | |
| Guanidinium isothiocyanate | 1.41 × 105± 1.23 × 104 | 1.92 × 105± 1.70 × 104 | 3.41 × 104± 3.65 × 103 |
| 31.3 ± 0.16 | 30.9 ± 0.07 | 33.3 ± 1.59 | |
| Proteinase K | 2.99 × 105± 2.88 × 104 | 2.21 × 105± 2.97 × 104 | 3.59 × 105± 5.10 × 104 |
| 30.5 ± 0.19 | 30.3 ± 0.38 | 29.6 ± 0.38 | |
| NaI | 2.51 × 105± 2.91 × 104 | 2.12 × 105± 3.01 × 104 | 9.12 × 104± 9.65 × 103 |
| 30.7 ± 0.17 | 30.5 ± 0.66 | 31.4 ± 0.14 | |
| NaOH lysis | 3.49 × 108± 2.72 × 107 | 2.08 × 106± 2.80 × 105 | 4.40 × 107± 2.06 × 106 |
| 19.9 ± 0.18 | 30.0 ± 0.52 | 26.2 ± 0.78 | |
| Alkaline lysis | N/ N | N/ N | N/ N |
| Boiling | 2.14 × 108± 2.16 × 107 | 1.83 × 106± 1.05 × 105 | 1.48 × 107± 1.22 × 106 |
| 23.9 ± 0.10 | 30.1 ± 0.69 | 27.4 ± 0.94 | |
Table 3 indicated the copy number and Ct values of 8 positive HBV serum samples based on the optimized primers and TaqMan probe as well as DNA extraction. Their SD and CV unfolded that quantification of hepatitis B virus DNA in triplicate by real-time PCR was reliable and accurate.
| Sample | Ct | Copy number /mL | ||
| Mean ± SD | CV (%) | Mean ± SD | CV (%) | |
| 1 | 25.4 ± 0.24 | 0.9 | 1.4 × 109± 2.38 × 108 | 17.0 |
| 2 | 31.9 ± 0.20 | 0.6 | 1.4 × 107± 1.87 × 106 | 13.4 |
| 3 | 31.5 ± 0.52 | 1.7 | 1.8 × 107± 2.32 × 106 | 12.9 |
| 4 | 27.3 ± 0.14 | 0.5 | 3.7 × 108± 3.73 × 107 | 10.0 |
| 5 | 34.0 ± 0.27 | 8.1 | 6.4 × 106± 6.08 × 105 | 9.5 |
| 6 | 32.3 ± 0.52 | 0.2 | 1.1 × 107± 4.43 × 106 | 4.0 |
| 7 | 27.0 ± 0.23 | 0.8 | 4.6 × 108± 7.22 × 106 | 15.7 |
| 8 | 33.9 ± 0.12 | 0.4 | 3.1 × 106± 2.76 × 105 | 8.9 |
Detection and analysis of PCR products should be carried out simultaneously with temperature cycling during amplification. If the fundamental properties of DNA, such as product size, quantity, sequence, or melting profile can specifically identify the products during PCR, no further analysis is required.
Real-time PCR is a powerful diagnostic tool capable of rapidly generating reliable and reproducible results with reduced risks of cross contamination[29]. The amplification efficiency can be detected with fluorescent probes. All steps are accomplished automatically by computer except for sample preparation, which can monitor PCR reaction timely.
To make our assay more sensitive and efficient, we optimized three pairs of primers and probes targeting the conserved S, C and X regions of HBV genome respectively. The highest copy number and Ct value of HBV serum samples were observed on the standard curve originated from S region. This indicated that the sensitivity of real-time PCR can be improved by adjusting primes and probe[21].
HBV DNA extraction from serum is a key step in real-time PCR, as it directly affects the accuracy of quantification of viral load. NaOH lysis showed that the highest copy number of HBV serum samples A, B, and C was 3.49 × 108/mL, 2.08 × 106/mL and 4.40 × 107/mL respectively. Boiling was as effective as NaOH lysis in DNA extraction from HBV serum samples A, B, and C. The copy number of HBV serum samples A, B, and C detected by boiling was 2.14 × 108/mL, 1.83 × 106/mL, and 1.48 × 107/mL, respectively. However, boiling was unsuitable for DNA extraction from serum with high viscidity[28].
Compared with the methods of NaOH lysis and boiling, guanidinium isothiocyanate, proteinase K and NaI decreased the copy number of HBV serum samples A, B and C to approximately 102-103/mL. Proteinase K lysis was a bit better than the other two methods. In all three methods, the lysis was mixed with serum and extracted with chloroform/isoamyl alcohol (24:1), followed by precipitation and washing with absolute alcohol. A large quantity of DNA was lost due to the complicated process of these methods. DNA extraction with alkaline failed to detect serum DNA in our experiment, possibly due to the high concentration and pH value. On the contrary, 0.4 mol/L NaOH lysis followed by neutralization with 0.4 mol/L Tris-HCI (pH7.6) showed excellent efficacy because of a moderate alkaline concentration and lower pH value which may significantly improve the efficiency of PCR amplification.
In conclusion, real-time PCR based on the optimized primers, probe and DNA extraction is a simple, accurate, specific and sensitive method for the measurement of HBV viral load in serum. NaOH lysis for HBV serum DNA extraction is rapid, simple and efficient, making the assay suitable for handling a large number of clinical HBV serum samples. Since there are about 200 million HBV carriers in China, doctors need to know the HBV status of patients before starting any medical treatment. This assay may be especially useful for monitoring the therapeutic effects in chronically infected patients on antiviral therapy.
We thank Professor Xian-Rang Song from Shandong Cancer Hospital for his technical support.
S- Editor Liu Y L- Editor Wang XL E- Editor Ma WH
| 1. | Bai YJ, Zhao JR, Lv GT, Zhang WH, Wang Y, Yan XJ. Rapid and high throughput detection of HBV YMDD mutants with fluorescence polarization. World J Gastroenterol. 2003;9:2344-2347. [PubMed] |
| 2. | Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1712] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 3. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1734] [Cited by in RCA: 1750] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 4. | Gitlin N. Hepatitis B: diagnosis, prevention, and treatment. Clin Chem. 1997;43:1500-1506. [PubMed] |
| 5. | Jardi R, Rodriguez F, Buti M, Costa X, Cotrina M, Valdes A, Galimany R, Esteban R, Guardia J. Quantitative detection of hepatitis B virus DNA in serum by a new rapid real-time fluorescence PCR assay. J Viral Hepat. 2001;8:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Paraskevis D, Haida C, Tassopoulos N, Raptopoulou M, Tsantoulas D, Papachristou H, Sypsa V, Hatzakis A. Development and assessment of a novel real-time PCR assay for quantitation of HBV DNA. J Virol Methods. 2002;103:201-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Chan TM, Fang GX, Tang CS, Cheng IK, Lai KN, Ho SK. Preemptive lamivudine therapy based on HBV DNA level in HBsAg-positive kidney allograft recipients. Hepatology. 2002;36:1246-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 127] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1381] [Cited by in RCA: 1347] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 9. | Omata M. Treatment of chronic hepatitis B infection. N Engl J Med. 1998;339:114-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Payungporn S, Tangkijvanich P, Jantaradsamee P, Theamboonlers A, Poovorawan Y. Simultaneous quantitation and genotyping of hepatitis B virus by real-time PCR and melting curve analysis. J Virol Methods. 2004;120:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Yeh SH, Tsai CY, Kao JH, Liu CJ, Kuo TJ, Lin MW, Huang WL, Lu SF, Jih J, Chen DS. Quantification and genotyping of hepatitis B virus in a single reaction by real-time PCR and melting curve analysis. J Hepatol. 2004;41:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 176] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 12. | Chen RW, Piiparinen H, Seppänen M, Koskela P, Sarna S, Lappalainen M. Real-time PCR for detection and quantitation of hepatitis B virus DNA. J Med Virol. 2001;65:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Garson JA, Grant PR, Ayliffe U, Ferns RB, Tedder RS. Real-time PCR quantitation of hepatitis B virus DNA using automated sample preparation and murine cytomegalovirus internal control. J Virol Methods. 2005;126:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Ho SK, Yam WC, Leung ET, Wong LP, Leung JK, Lai KN, Chan TM. Rapid quantification of hepatitis B virus DNA by real-time PCR using fluorescent hybridization probes. J Med Microbiol. 2003;52:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Weinberger KM, Wiedenmann E, Böhm S, Jilg W. Sensitive and accurate quantitation of hepatitis B virus DNA using a kinetic fluorescence detection system (TaqMan PCR). J Virol Methods. 2000;85:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 16. | Weiss J, Wu H, Farrenkopf B, Schultz T, Song G, Shah S, Siegel J. Real time TaqMan PCR detection and quantitation of HBV genotypes A-G with the use of an internal quantitation standard. J Clin Virol. 2004;30:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Zanella I, Rossini A, Domenighini D, Albertini A, Cariani E. Quantitative analysis of hepatitis B virus DNA by real-lime amplification. Eur J Clin Microbiol Infect Dis. 2002;21:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 18. | Zhao JR, Bai YJ, Zhang QH, Wan Y, Li D, Yan XJ. Detection of hepatitis B virus DNA by real-time PCR using TaqMan-MGB probe technology. World J Gastroenterol. 2005;11:508-510. [PubMed] |
| 19. | Qian WP, Tan YQ, Chen Y, Peng Y, Li Z, Lu GX, Lin MC, Kung HF, He ML, Shing LK. Rapid quantification of semen hepatitis B virus DNA by real-time polymerase chain reaction. World J Gastroenterol. 2005;11:5385-5389. [PubMed] |
| 20. | Holland PM, Abramson RD, Watson R, Gelfand DH. Detection of specific polymerase chain reaction product by utilizing the 5'----3' exonuclease activity of Thermus aquaticus DNA polymerase. Proc Natl Acad Sci USA. 1991;88:7276-7280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1859] [Cited by in RCA: 1679] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 21. | Proudnikov D, Yuferov V, Zhou Y, LaForge KS, Ho A, Kreek MJ. Optimizing primer--probe design for fluorescent PCR. J Neurosci Methods. 2003;123:31-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Andolfatto S, Namour F, Garnier AL, Chabot F, Gueant JL, Aimone-Gastin I. Genomic DNA extraction from small amounts of serum to be used for alpha1-antitrypsin genotype analysis. Eur Respir J. 2003;21:215-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Smith K, Diggle MA, Clarke SC. Comparison of commercial DNA extraction kits for extraction of bacterial genomic DNA from whole-blood samples. J Clin Microbiol. 2003;41:2440-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Kok T, Wati S, Bayly B, Devonshire-Gill D, Higgins G. Comparison of six nucleic acid extraction methods for detection of viral DNA or RNA sequences in four different non-serum specimen types. J Clin Virol. 2000;16:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Lin Z, Floros J. Genomic DNA extraction from small amounts of sera to be used for genotype analysis. Biotechniques. 1998;24:937-940. [PubMed] |
| 26. | Lin Z, Floros J. Protocol for genomic DNA preparation from fresh or frozen serum for PCR amplification. Biotechniques. 2000;29:460-42, 464, 466. [PubMed] |
| 27. | Manzin A, Salvoni G, Bagnarelli P, Menzo S, Carloni G, Clementi M. A single-step DNA extraction procedure for the detection of serum hepatitis B virus sequences by the polymerase chain reaction. J Virol Methods. 1991;32:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Sandford AJ, Paré PD. Direct PCR of small genomic DNA fragments from serum. Biotechniques. 1997;23:890-892. [PubMed] |
| 29. | Mackay IM. Real-time PCR in the microbiology laboratory. Clin Microbiol Infect. 2004;10:190-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 448] [Article Influence: 21.3] [Reference Citation Analysis (0)] |