Published online Dec 7, 2006. doi: 10.3748/wjg.v12.i45.7271
Revised: June 28, 2006
Accepted: November 6, 2006
Published online: December 7, 2006
AIM: To evaluate the impact of hepatitis C virus (HCV) infection with genotype 1 or 3 and the presence or absence of liver cirrhosis (LC) in the early viral kinetics response to treatment.
METHODS: Naive patients (n = 46) treated with interferon-α (IFN-α) and ribavirin and followed up with frequent early HCV-RNA determinations were analysed. Patients were infected with genotype 1 (n = 28, 7 with LC) or 3 (n = 18, 5 with LC).
RESULTS: The first phase decline was larger in genotype 3 patients than in genotype 1 patients (1.72 vs 0.95 log IU/mL, P < 0.001). The second phase slope decline was also larger in genotype 3 patients than in genotype 1 patients (0.87 vs 0.15 log/wk, P < 0.001). Differences were found in both cirrhotic and non-cirrhotic patients. Genotype 1 cirrhotic patients had a slower 2nd phase slope than non-cirrhotic patients (0.06 vs 0.18 log/wk, P < 0.02). None of genotype 1 cirrhotic patients had a 1st phase decline larger than 1 log (non-cirrhotic patients: 55%, P < 0.02). A similar trend toward a slower 2nd phase slope was observed in genotype 3 cirrhotic patients but the 1st phase slope decline was not different. Sustained viral response was higher in genotype 3 patients than in genotype 1 patients (72% vs 14%, P < 0.001) and in genotype 1 non-cirrhotic patients than in genotype 1 cirrhotic patients (19% vs 0%). A second phase decline slower than 0.3 log/wk was predictive of non-response in all groups.
CONCLUSION: Genotype 3 has faster early viral decline than genotype 1. Cirrhosis correlates with a slower 2nd phase decline and possibly with a lower 1st phase slope decline in genotype 1 patients.
- Citation: Medeiros-Filho JE, Mello IMVGC, Pinho JRR, Neumann AU, Malta FM, Silva LCD, Carrilho FJ. Differences in viral kinetics between genotypes 1 and 3 of hepatitis C virus and between cirrhotic and non-cirrhotic patients during antiviral therapy. World J Gastroenterol 2006; 12(45): 7271-7277
- URL: https://www.wjgnet.com/1007-9327/full/v12/i45/7271.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i45.7271
Chronic hepatitis C virus (HCV) infection is a major health problem with about 170 million infected subjects worldwide[1]. It is the most common cause of liver cirrhosis (LC), hepatocellular carcinoma, end-stage liver failure, and liver transplantation around the world. Achieving a sustained viral response (SVR), which is defined as undetectable HCV-RNA in serum 24 wk after the end of treatment, is the most effective way to prevent disease progression. Interferon-alpha (IFN-α) plus ribavirin (RBV) therapy (combined therapy) achieves an overall response rate ranging from 42% to 82% in naïve patients[2]. Treatment outcome is highly influenced by dosage and IFN-α presentation, use of RBV, and HCV genotype. For HCV genotype 1-infected patients, optimal SVR rates are achieved with pegylated IFN-α plus RBV for 48 wk (a SVR rate of 40%-55%)[3]. For patients infected with HCV genotypes 2 and 3, a high SVR rate (65%-80%) is observed with standard IFN-α and RBV treatment for 24 wk[4]. Various baseline factors, including histological status and the presence of cirrhosis also affect the outcome of treatment[5].
Hepatitis C viral kinetics has provided an explanation for the differential response among the different genotypes and allows to evaluate the response rates early during therapy with conventional or pegylated interferons[6]. The effects of treatment on the replication of HCV genotype 1 and the clearance of free virions and infected cells have been investigated in previous studies of viral kinetics[7-9]. Blocking virion production from infected cells with daily IFN-α treatment results in a biphasic viral decline pattern[8] which can predict the treatment outcome[10,11]. Studies of viral kinetics in patients infected with HCV genotype 2 showed that IFN-α treatment is more effective in blocking viral production, and results in faster clearance of virions and infected cells[12,13]. Genotype 4 viral dynamics parameters appear similar to HCV-1 and are slower than those of HCV-2 and HCV-3 infected patients, who should be grouped with those with HCV-1 infection when therapeutic schemes are considered in relation to genotype[14]. Other baseline parameters, such as viral load before treatment, race, gender, and age, have been studied for their effect on HCV viral kinetics[11,15].
However, viral kinetics and the effect of cirrhotic status on viral kinetics in patients infected with HCV genotype 3 have not been extensively studied yet. In a previous report analysing the viral kinetics response to standard or pegylated interferon α2a, HCV-3 patients were grouped as “HCV non-1” with HCV-2, -4 and -5 patients and compared to HCV-1 infected patients[16]. Genotype 3 deserves special attention as it is particularly common in some regions of the world, such as South and South East Asia[17], Australia[18] and Brazil[19], as well as in intravenous drug users in Europe[20].
In this study, considering the frequency of HCV-3 infection in our country, we compared the viral kinetics of early response to treatment by taking frequent samples from naïve patients infected with HCV genotypes 1 and 3. Furthermore, we assessed the effects of LC in the early viral kinetics.
Forty-six naïve chronic hepatitis C patients from the Hepatology Branch, Department of Gastroenterology, University of São Paulo School of Medicine, São Paulo, Brazil were enrolled. Inclusion criteria were patients infected with HCV genotype 1 or 3 who did not undergo previous treatment, with an age of 18 to 70 years, those with their viraemia detectable by reverse transcriptase-polymerase chain reaction (RT-PCR) and their alanine aminotransferase (ALT) serum levels being 1.5 times the upper normal limit, and those having no other significant pathological findings. Histological activity grade and fibrosis stage were evaluated according to the METAVIR scoring system[21]. In particular, cirrhosis was considered when stage F4 was detected. Normal hepatic function and a liver biopsy within six months before treatment demonstrating necroinflammatory stage A2 or A3 were required. Standard inclusion and exclusion criteria for chronic hepatitis C treatment with IFN and ribavirin were applied. Written informed consent was obtained from all patients. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Ethical Committee of the University of São Paulo School of Medicine and the Instituto Adolfo Lutz.
Patients were randomised into different IFN-α treatment schedule groups (Table 1). All patients received conventional IFN-α (Roferon®, F. Hoffmann-La Roche Ltd., Basel, Switzerland) to allow a better comparison among the kinetic parameters, but the treatment was continued for 48 wk to allow a better response in all the studied groups. All patients received a first dose of 9 Mega units (MU) of IFN-α. Group “3 TIW” (11 patients) received additional 3 MU doses of IFN-α three times a week until wk 48, while group “3 Q2D” (10 patients) received the same dose every other day. Group “3 QD” (13 patients) received 3 MU daily after the first dose until d 4, followed by 3 MU every other day until wk 48. Finally, group “9 QD” (12 patients) received 9 MU daily after the first dose until d 14, followed by 3 MU every other day until the end of treatment (48 wk). All groups received two doses of 500 mg ribavirin daily for 48 wk.
| Group | d 0 | d 1 | d 2 | d 3 | d 4 | d 5 | d 6 | d 7 | d 8 | d 9 | d 10 | d 11 | d 12 | d 13 | d 14 | d 15 | d 16 | |
| 3 TIW | 9 | - | 3 | - | 3 | - | - | 3 | - | 3 | - | 3 | - | - | 3 | - | 3 | 3 TIW until wk 48 |
| 3 Q2D | 9 | - | 3 | - | 3 | - | 3 | - | 3 | - | 3 | - | 3 | - | 3 | - | 3 | 3 Q2D until wk 48 |
| 3 QD | 9 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | - | 3 | |
| 9 QD | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | - | 3 |
Serum samples were collected just before IFN-α injections (with a tolerance of 15 min) in the first 2 wk and then every other day until d 28. Samples were processed within 2 h after collection and stored at -80°C.
HCV viral loads were determined using a commercially available quantitative assay (COBAS AMPLICOR MONITORTM 2.0, lower limit of detection 600 IU/mL, upper limit of linear quantitation 850 000 IU/mL, Roche Molecular Systems, CA, USA). Samples below the limit of detection were subjected to a qualitative PCR assay (AMPLICORTM, limit of detection 50 IU/mL, Roche Molecular Systems, CA, USA) while samples above the upper limit of detection were retested at 10 or 100 fold dilution in normal serum. Viral load determinations were performed at the same time using the same lot of the kit for all patients. Viral load results were transformed to log10.
Viral RNA was extracted from 100 μL of serum using guanidine isothiocyanate-phenol-chloroform as previously described[22]. The 5’ untranslated region (5’ UTR) of HCV genome was amplified, PCR products were subjected to cycle sequencing reactions using dideoxynucleotides (ddNTPs) with fluorescent markers (Kit ABI PrismR BigDyeTM terminator cycle sequencing ready reaction, Applied Biosystems, Foster City, CA, USA). Genotyping was carried by aligning both sequenced strands (sense and antisense), obtaining a consensus sequence and comparing this consensus sequence to a database[19].
Kinetic data were analysed using a previously published mathematical model[8], which assumes that there is a pre-treatment steady state and that the primary effect of IFN-α is to block viral production from infected cells. The first phase decline was defined as the log decline in viral load in the first 24 h, which was equal for all dosing schedule groups. The second phase slope decline was calculated from d 4 (96 h after the first injection) to d 14 to avoid effects of the transient rebound observed after switching from the 9 MU dose to the 3 MU dose on d 2 and differences between the study arms.
The statistical significance of differences between groups was assessed with the Mann-Withney non-parametric U-test for the distribution of quantitative variables and the Fisher’s exact test for the categorical variables. Multi-variable analysis was not performed since the small number of patients per sub-group did not allow any statistical power. P < 0.05 was considered statistically significant.
Forty-six HCV infected patients were included in this study. Twenty-eight of the patients were infected with genotype 1 virus, 7 of them (24%) were cirrhotic. Eighteen patients were infected with genotype 3 virus, 5 of them (27.7%) were cirrhotic. Four different IFN dosing schedules were tested (Table 1). There was no difference in baseline viral load (mean 5.9 log IU/mL) between the genotypes.
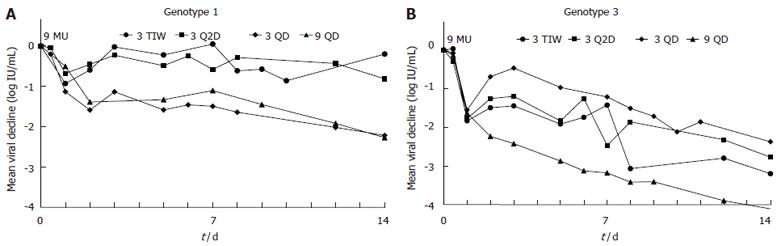
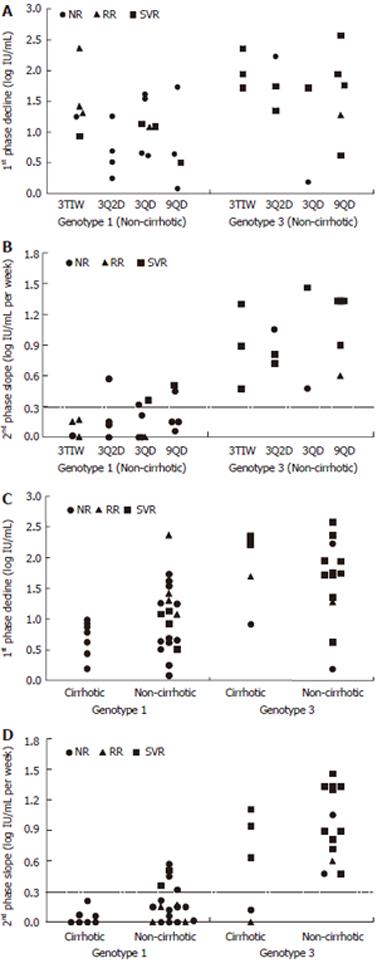
For non-cirrhotic patients, the kinetics of viral decline for each HCV genotype and treatment schedule is shown in Figure 1. As seen in this figure, oscillations in the viral load were found in patients of groups 3 TIW and 3 Q2D. Each treatment group had particular viral kinetic patterns in accordance with the IFN-α schedule. However, the decline was faster in genotype 3 patients than in genotype 1 patients (Table 2, Figures 2A and 2B). In patients without cirrhosis, the first phase decline after the first 9 MU dose which was given for all groups together, was significantly (P < 0.001) larger in genotype 3 patients (1.65 log) than in genotype 1 patients (1.03 log). Only 2 (15%) out of the 13 patients with genotype 3 had a 1st phase decline less than 1 log, compared with 9 (43%) out of the 21 patients with genotype 1. Also the decline of the second phase slope was significantly (P < 0.001) faster in genotype 3 patients (0.99 log per week) than in genotype 1 patients (0.18 log per week). None of the genotype 3 patients had a slope slower than 0.3 log per week compared with 16 out of the 21 (76%) genotype 1 patients.
The effect of different treatment schedules on viral kinetics was also compared separately between the two genotypes (Table 2, Figures 2A and 2B). As expected there was no difference in the 1st phase decline between the treatment groups since the treatment on the 1st day was the same for all patients. Also for the 2nd phase slope, no significant difference was found in the treatment groups, although there was a trend toward faster decline with the daily dose schedules (3 QD and 9 QD) in genotype 1 patients. Indeed, among non-cirrhotic genotype 1 patients receiving daily IFN-α (3 QD and 9 QD), 4 (36%) out of 11 patients had a slope faster than 0.3 log/wk as compared with 1 out of 10 (10%) in the 3 TIW or 3 Q2D group. In each treatment group, genotype 3 patients had a faster second phase slope than genotype 1 patients, with a statistical significance for the 9 QD group even with a small number of patients (Table 2).
Lastly, a lower viral load at the end of the first phase was strongly correlated with a faster second phase slope in all patients (P < 0.001; r = 0.6).
The impact of cirrhosis status on cirrhotic and non-cirrhotic patients was addressed by comparing parameters of viral kinetics for each HCV genotype. Patients of all treatment groups were analysed together since there was no significant difference in viral kinetic parameters between the interferon treatment schedules. There was no difference in baseline viral load between cirrhotic (5.9 log) and non-cirrhotic (6.0 log) genotype 1 patients (Table 3). Interestingly, genotype 3 cirrhotic patients had a significantly (P < 0.03) higher baseline viral load (6.4 log) than non-cirrhotic (5.7 log) genotype 3 patients and genotype 1 patients (Table 3).
A lower first phase decline was observed in genotype 1 cirrhotic patients (0.69 log) than in non-cirrhotic patients (1.03 log), but the difference was not statistically significant (Table 3). Nevertheless, as shown in Figure 2C, none (0%) of genotype 1 cirrhotic patients had a first-phase decline larger than 1 log IU/mL, compared with 55% of the non-cirrhotic genotype 1 patients (P < 0.02). Furthermore, a significantly (P < 0.02) slower second-phase decline was observed in genotype 1 cirrhotic patients (0.06 log IU/mL per week) when compared with non-cirrhotic patients (0.18 log per week) (Table 3). None of the cirrhotic genotype 1 patients had a slope faster than 0.3 log per week compared with 5 (24%) of 21 non-cirrhotic genotype 1 patients (Figure 2D).
As shown in Table 3, no difference in first phase decline was observed in genotype 3 patients as a function of cirrhosis status (1.89 vs 1.65 log IU/mL, P < 0.05). However, cirrhotic patients with the genotype 3 virus did have a trend towards a slower second-phase decline (0.57 log IU/mL per week) compared with non-cirrhotic patients (0.99 log per week), and notably the only 2 genotype 3 patients with a flat second phase were cirrhotic patients (Figure 2D).
Cirrhotic patients (Table 3) infected with genotype 1 virus had a less intense first phase (P < 0.01) and a slower second phase slope (P < 0.01) when compared with genotype 3 infected patients. When cirrhotic and non-cirrhotic patients were pooled together the differences in the genotypes became even more significant (Table 3).
Only 4 (14%) of 28 genotype 1 patients achieved a SVR. In contrast, 13 (72%) of 18 genotype 3 patients (P < 0.001) achieved a SVR (Table 4 and Figure 2). The difference in the genotypes was also seen between cirrhotic and non-cirrhotic patients.
The 4 (19%) of 21 genotype 1-infected patients who achieved a SVR were non-cirrhotic. In contrast, none (0%) of cirrhotic patients achieved a SVR. Interestingly, all the patients who relapsed were also non-cirrhotic (none of the cirrhotic patients achieved a SVR). On the other hand, 3 (60%) out of 5 cirrhotic genotype 3 patients achieved a SVR, which was comparable with 77% of non-cirrhotic genotype 3 patients. Only 1 genotype 3 patient was a true non-responder. A cirrhotic patient and 2 non-cirrhotic non-responders discontinued their treatment early (Table 4 and Figure 2).
Lastly, none of the patients with a second phase slope slower than 0.3 log IU/mL per week, irrespective of genotype, histology status or treatment group, achieved a sustained virological response (negative predictive value = 100%, Figure 2).
Hepatitis C viral kinetics has become an important tool both for investigating the effects of drugs on HCV viral replication[7-10,23,24] and for making clinical decisions about HCV treatment with interferon plus ribavirin therapy[2]. Previous studies have addressed the kinetics of HCV genotypes[7-10,12-16,23-25]. To our knowledge, only one study has addressed the viral kinetics of the early response in patients infected with genotype 3[25] and in that study, HCV-3 patients were grouped together with HCV-2, -4 and –5 patients and viral kinetic data were shown for the “HCV non-1” patients. Our results clearly showed that the 1st and 2nd phase declines were faster in genotype 3 patients than in genotype 1 patients, which were independent of the treatment schedule used or the cirrhosis status when compared to HCV-1. The data relavant to geographic areas were genotypes 1 and 3 which are the most frequent in Brazil, Australia, South and South East Asia, etc. To our knowledge, this is the first study to evaluate the effect of LC on viral kinetic parameters.
As conventional IFN-α is still used in many regions of the world particularly in developing countries due to its lower cost, we consider that these data are always valuable for understanding the viral response to conventional IFN-α as the response to treatment of genotype 3 patients is similar to the response to pegylated IFN-α. To ensure a better comparison to genotype 1-infected patients, they were also treated with standard IFN-α, but a 48 wk course was chosen to ensure a better response among all the studied patients in our study.
These results agree with clinical trials of IFN-α (standard or pegylated) and ribavirin treated patients, demonstrating that SVR rates are significantly higher in genotype 3 HCV-infected patients than in genotype 1-infected patients[2,4,5,12]. These results are also consistent with the differences found in viral kinetics between genotype 1 and 2 patients[12,13], as it is known that genotypes 2 and 3 have a similar response rate. We found that the effectiveness in blocking viral production was higher in genotype 3 and the rate of loss of infected cells was faster. Unfortunately, the frequency of sampling in the first 2 d in our study was not enough to study the clearance of free viruses. A detailed comparison between viral kinetics of genotypes 2 and 3 is still of interest.
We observed in this study an intriguing higher baseline viral load in genotype 3 cirrhotic patients than in genotype 3 non-cirrhotic patients or in genotype 1 patients in general. This result was not demonstrated in genotype 1 cirrhotic patients and non-cirrhotic patients, or in genotype 1 or 3 non-cirrhotic patients. The difference observed in genotype 3 cirrhotic patients may be related to the higher steatosis found in these patients[26].
One of the most important results of this study is the observed effect of liver histology on viral kinetics. We have clearly demonstrated that LC adversely impacts early viral kinetics, especially the slope of the second phase viral decline. This is the first viral kinetics study demonstrating a significant difference in the second phase, which is related to the rate of loss of infected cells and is predictive of SVR as a function of the histological status of the liver. The slower decline in cirrhotic patients, which is statistically significant in genotype 1 and genotype 3, can explain the sub-optimal results obtained with interferon and ribavirin in this difficult-to-treat subgroup, even with pegylated interferons[5]. The mechanism behind this effect may be due to a lower immune infiltration to all parts of the liver and/or a lower potency of the immune response to clear infected cells in the cirrhotic environment. Further experimental studies are needed to clarify the mechanism.
On the other hand, our observation that the first phase decline was limited in genotype 1 patients with cirrhosis, but not in genotype 3 patients, was unexpected. The mechanism behind this observation is not understood. Since resistance to IFN-α can be viral or cellular, a possible hypothesis is that hepatocytes in a cirrhotic liver of HCV genotype 1 patients are more resistant to IFN-α, which is different from cirrhosis due to genotype 3 that is strongly related to steatosis[25-28]. Steatosis among genotype 3-infected patients does not appear to be related to the presence of HCV antigens within single hepatocytes but to indirect mechanisms, possibly mediated by cytokine[29]. Two HCV proteins, core and NS5A seem to be sufficient to induce lipid accumulation in hepatocytes[30,31], especially in genotype 3[32]. Steatosis is associated with the development of fibrosis[29], which is related to genotype 3[26] and may accelerate fibrosis progression. In genotype 3 patients, steatosis can regress when HCV replication is inhibited supporting a cytophatic effect of genotype 3[33], but the mechanisms underlying the influence of steatosis in response remain to be determined.
In summary, infection with genotype 3 or genotype 1 virus is a more important factor in determining viral kinetics than cirrhosis status or IFN-α treatment schedule. However, within genotypes 1 and 3, patients with cirrhosis have a slower 2nd phase decline, and among genotype 1 patients the 1st phase has a trend toward a lower decline. Thus, advanced histological status although multi-factorial, has a significant adverse effect on viral kinetics, especially in genotype 1 cirrhotic patients. Our results suggest that both genotype 1 and 3 patients with cirrhosis may need more aggressive treatment than the current standard treatment for each genotype. In our study, early prediction of sustained viral response using a second phase slope slower than 0.3 log per week had a negative predictive value of 100%, which is in accordance with previous studies [6]. Thus early viral kinetic prediction can work for cirrhotic patients, since the net effects of all factors are summarized into the first and second phases of viral kinetics.
S- Editor Wang GP L- Editor Wang XL E- Editor Ma WH
| 1. | Afdhal NH. The natural history of hepatitis C. Semin Liver Dis. 2004;24 Suppl 2:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 200] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 2. | NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19:1-46. [PubMed] |
| 3. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4736] [Cited by in RCA: 4558] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 4. | McHutchison JG, Poynard T. Combination therapy with interferon plus ribavirin for the initial treatment of chronic hepatitis C. Semin Liver Dis. 1999;19 Suppl 1:57-65. [PubMed] |
| 5. | Heathcote EJ, Shiffman ML, Cooksley WG, Dusheiko GM, Lee SS, Balart L, Reindollar R, Reddy RK, Wright TL, Lin A. Peginterferon alfa-2a in patients with chronic hepatitis C and cirrhosis. N Engl J Med. 2000;343:1673-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 605] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 6. | Ferenci P. Predicting the therapeutic response in patients with chronic hepatitis C: the role of viral kinetic studies. J Antimicrob Chemother. 2004;53:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Zeuzem S, Schmidt JM, Lee JH, Rüster B, Roth WK. Effect of interferon alfa on the dynamics of hepatitis C virus turnover in vivo. Hepatology. 1996;23:366-371. [PubMed] |
| 8. | Neumann AU, Lam NP, Dahari H, Gretch DR, Wiley TE, Layden TJ, Perelson AS. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1604] [Cited by in RCA: 1451] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 9. | Lam NP, Neumann AU, Gretch DR, Wiley TE, Perelson AS, Layden TJ. Dose-dependent acute clearance of hepatitis C genotype 1 virus with interferon alfa. Hepatology. 1997;26:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 260] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Layden JE, Layden TJ, Reddy KR, Levy-Drummer RS, Poulakos J, Neumann AU. First phase viral kinetic parameters as predictors of treatment response and their influence on the second phase viral decline. J Viral Hepat. 2002;9:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Layden TJ, Layden JE, Reddy KR, Levy-Drummer RS, Poulakos J, Neumann AU. Induction therapy with consensus interferon (CIFN) does not improve sustained virologic response in chronic hepatitis C. J Viral Hepat. 2002;9:334-339. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Neumann AU, Lam NP, Dahari H, Davidian M, Wiley TE, Mika BP, Perelson AS, Layden TJ. Differences in viral dynamics between genotypes 1 and 2 of hepatitis C virus. J Infect Dis. 2000;182:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 160] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Kohara M, Tanaka T, Tsukiyama-Kohara K, Tanaka S, Mizokami M, Lau JY, Hattori N. Hepatitis C virus genotypes 1 and 2 respond to interferon-alpha with different virologic kinetics. J Infect Dis. 1995;172:934-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Halfon P, Neumann AU, Bourlière M, Rieu A, Chadapaud S, Khiri H, Ouzan D, Cacoub P. Slow viral dynamics of hepatitis C virus genotype 4. J Viral Hepat. 2003;10:351-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Layden-Almer JE, Ribeiro RM, Wiley T, Perelson AS, Layden TJ. Viral dynamics and response differences in HCV-infected African American and white patients treated with IFN and ribavirin. Hepatology. 2003;37:1343-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Zeuzem S, Herrmann E, Lee JH, Fricke J, Neumann AU, Modi M, Colucci G, Roth WK. Viral kinetics in patients with chronic hepatitis C treated with standard or peginterferon alpha2a. Gastroenterology. 2001;120:1438-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 199] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Simmonds P. Genetic diversity and evolution of hepatitis C virus--15 years on. J Gen Virol. 2004;85:3173-3188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 632] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 18. | Kaba S, Dutta U, Byth K, Crewe EB, Khan MH, Coverdale SA, Lin R, Liddle C, Farrell GC. Molecular epidemiology of hepatitis C in Australia. J Gastroenterol Hepatol. 1998;13:914-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Campiotto S, Pinho JR, Carrilho FJ, Da Silva LC, Souto FJ, Spinelli V, Pereira LM, Coelho HS, Silva AO, Fonseca JC. Geographic distribution of hepatitis C virus genotypes in Brazil. Braz J Med Biol Res. 2005;38:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 126] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Pawlotsky JM, Tsakiris L, Roudot-Thoraval F, Pellet C, Stuyver L, Duval J, Dhumeaux D. Relationship between hepatitis C virus genotypes and sources of infection in patients with chronic hepatitis C. J Infect Dis. 1995;171:1607-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 228] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3081] [Article Influence: 106.2] [Reference Citation Analysis (0)] |
| 22. | Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40518] [Cited by in RCA: 39097] [Article Influence: 1028.9] [Reference Citation Analysis (0)] |
| 23. | Nakajima H, Shimomura H, Iwasaki Y, Ikeda F, Umeoka F, Chengyu P, Taniguchi H, Ohnishi Y, Takagi SJ, Fujioka S. Anti-viral actions and viral dynamics in the early phase of three different regimens of interferon treatment for chronic hepatitis C: differences between the twice-daily administration of interferon-beta treatment and the combination therapy with interferon-alpha plus ribavirin. Acta Med Okayama. 2003;57:217-225. [PubMed] |
| 24. | Torre F, Giusto R, Grasso A, Brizzolara R, Campo N, Sinelli N, Balestra V, Picciotto A. Clearance kinetics of hepatitis C virus under different antiviral therapies. J Med Virol. 2001;64:455-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Zeuzem S, Pawlotsky JM, Lukasiewicz E, von Wagner M, Goulis I, Lurie Y, Gianfranco E, Vrolijk JM, Esteban JI, Hezode C. International, multicenter, randomized, controlled study comparing dynamically individualized versus standard treatment in patients with chronic hepatitis C. J Hepatol. 2005;43:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Rubbia-Brandt L, Fabris P, Paganin S, Leandro G, Male PJ, Giostra E, Carlotto A, Bozzola L, Smedile A, Negro F. Steatosis affects chronic hepatitis C progression in a genotype specific way. Gut. 2004;53:406-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 206] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 27. | Asselah T, Rubbia-Brandt L, Marcellin P, Negro F. Steatosis in chronic hepatitis C: why does it really matter. Gut. 2006;55:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 288] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 28. | Abid K, Pazienza V, de Gottardi A, Rubbia-Brandt L, Conne B, Pugnale P, Rossi C, Mangia A, Negro F. An in vitro model of hepatitis C virus genotype 3a-associated triglycerides accumulation. J Hepatol. 2005;42:744-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Grassi A, Ballardini G, Susca M, Bianchini F, Bonoli S, Bianchi FB, Lenzi M. HCV liver infection and liver steatosis: evidence for indirect mechanisms in genotype 3. Aliment Pharmacol Ther. 2005;22 Suppl 2:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Barba G, Harper F, Harada T, Kohara M, Goulinet S, Matsuura Y, Eder G, Schaff Z, Chapman MJ, Miyamura T. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc Natl Acad Sci USA. 1997;94:1200-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 499] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 31. | Shi ST, Polyak SJ, Tu H, Taylor DR, Gretch DR, Lai MM. Hepatitis C virus NS5A colocalizes with the core protein on lipid droplets and interacts with apolipoproteins. Virology. 2002;292:198-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 239] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 32. | Asselah T, Boyer N, Guimont MC, Cazals-Hatem D, Tubach F, Nahon K, Daïkha H, Vidaud D, Martinot M, Vidaud M. Liver fibrosis is not associated with steatosis but with necroinflammation in French patients with chronic hepatitis C. Gut. 2003;52:1638-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Rubbia-Brandt L, Giostra E, Mentha G, Quadri R, Negro F. Expression of liver steatosis in hepatitis C virus infection and pattern of response to alpha-interferon. J Hepatol. 2001;35:307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |