Published online Nov 28, 2006. doi: 10.3748/wjg.v12.i44.7183
Revised: October 8, 2006
Accepted: October 11, 2006
Published online: November 28, 2006
AIM: To evaluate the efficacy of dipstick test in diagnosis of spontaneous bacterial peritonitis (SBP) in cirrhotic patients who underwent abdominal paracentesis based on the locally available dipstick test.
METHODS: There were 200 consecutive samples from cirrhotic patients who underwent abdominal paracentesis. Urine dipstick (Combur10 Test®M, Roche, Mannheim, Germany) was used as a screening test. A manual cell count with differential study was done in all samples by experienced technicians. The polymorphonuclear (PMN) cell count more than 250 cells/mm3 was used as a diagnostic cut off level. One to three plus dipstick results were used as cut off levels for a positive result. The dipstick test results had to be agreed by three experienced readers. The sensitivity, specificity, positive and negative predictive values and accuracy of two different colorimetric cut off scales (1+ and 2+) were calculated and compared.
RESULTS: The prevalence of SBP diagnosed by manual cell count was 21.0%. There were 128 specimens that had a true negative result by dipstick. The sensitivity, specificity, positive and negative predictive values and accuracy of 1+ and 2+ cut off scale to diagnose SBP were 88%, 81%, 55%, 96% and 83% respectively, and 63%, 96%, 82%, 81% and 89% respectively.
CONCLUSION: Dipstick test can be used as a rapid test for screening of SBP. The higher cut off colorimetric scale has a better specificity and positive predictive value but a lower sensitivity.
- Citation: Rerknimitr R, Rungsangmanoon W, Kongkam P, Kullavanijaya P. Efficacy of leukocyte esterase dipstick test as a rapid test in diagnosis of spontaneous bacterial peritonitis. World J Gastroenterol 2006; 12(44): 7183-7187
- URL: https://www.wjgnet.com/1007-9327/full/v12/i44/7183.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i44.7183
Apart from variceal bleeding, spontaneous bacterial peritonitis (SBP) is another serious complication that can develop in cirrhotic patients. Prompt diagnosis and treatment are essential for the survival of patients with SBP[1,2]. Unfortunately, symptoms of SBP including fever, abdominal pain, nausea, and vomiting are not presented in all cirrhotic patients who develop SBP[3,4]. In addition, many hospitalized cirrhotic patients develop SBP during their non-SBP related admissions especially those with gastrointestinal bleeding[5]. Therefore, routine diagnostic paracentesis is the recommended practice for patients with ascites who develop signs or risk factors for SBP. The standard criteria for diagnosis of SBP are an ascitic fluid polymorphoneuclear (PMN) cell count of ≥ 250/mm3 and/or a positive ascitic fluid bacterial culture[6-8]. Due to the nature of bacterial culture, the result is not available within a day. Therefore, decision making for SBP treatment is mainly based on PMN cell count. However, manual ascitic fluid PMN cell count is not always available in some small patient care units especially in the out patient offices and sometimes it cannot be done as in emergency basis.
Recently, leukocyte esterase activity testing by dipstick has been used for a rapid diagnosis of infection in many body fluids such as urine, pleural fluid, and cerebrospinal fluid[9-11]. The leukocyte esterase released from PMN cells reacts with an esterified chemical compound in the reagent strip yielding a violet azo dye, the intensity of which correlates to leukocyte count[12]. Recently, many studies have shown the efficacy of dipstick in diagnosing SBP[13-19]. However, strip tests used in these studies are not the same and have different colorimetric scales. Therefore, the sensitivity and accuracy of different dipsticks may vary. To date, there has been no recommended calorimetric scale for each dipstick to diagnose SBP.
The aim of this study was to evaluate the usefulness of dipstick in rapid diagnosis of SBP in cirrhotic patients who underwent abdominal paracentesis based on the locally available dipstick test and to define the validity scores from 2 different thresholds of colorimetric scales.
During the period between July 2004 and November 2005 at King Chulalongkorn Memorial Hospital, 200 consecutive ascitic fluid specimens (including 21 from out patient unit) were collected from 127 cirrhotic patients (Table 1). The clinical indications for paracentesis were routine paracentesis (n = 95), relief of patient’s discomfort (n = 54), suspected SBP (n = 13), miscellaneous (n = 38). The diagnosis of cirrhosis was established according to the histologic criteria or analytical, clinical, and ultrasonographic findings of the liver. Informed consent was obtained from all patients, and the study was approved by the Ethics Committee of the University.
| Characteristics | n (%) |
| Male/Female | 75/52 |
| Age (mean + SD) | 57.4 ± 13.3 |
| Child-Pugh classification A/B/C | 3/34/90 |
| Etiology of cirrhosis | |
| Hepatitis B | 34 (26.8) |
| Hepatitis C | 8 (6.3) |
| Alcohol | 25 (19.7) |
| Alcohol and hepatitis B | 9 (7.1) |
| Alcohol and hepatitis C | 9 (7.1) |
| Hepatitis B and hepatitis C | 1 (0.8) |
| Autoimmune | 2 (1.6) |
| Hemochromatosis | 2 (1.6) |
| Cryptogenic | 10 (7.9) |
| Others | 27 (21.3) |
Immediately after paracentesis, fresh ascitic fluid specimen was collected in a clean dry container and tested using a dipstick (Combur10 Test®M, Roche, Mannheim, Germany) for granulocyte esterase designed for urine analysis. The strip has a colorimetric 4-grade scale (negative, 1+ to 3+) and is read at 90 s for granulocyte esterase activity. The procedure was the same as what the manufacturer described for urine.
Each of the colorimetric scale readings was graded by 2 well trained on-duty internists (the reading processes were scored separately). All the readings were confirmed by WR who is a house staff covering for this project. WR made the final decision for all discrepancy results. A correlation between PMN cell count and the 4-grade scale was suggested by the manufacturer as follows: negative, 0 PMN cell/mm3; 1+, 10-25 PMN cells/mm3; 2+, 75 PMN cells/mm3; 3+, 500 PMN cells/mm3.
Ascitic fluid was conventionally processed including cytology, PMN cell count and lymphocyte count, and appropriate biochemical tests (glucose, protein, albumin, lactic dehydrogenase, and sugar). The sample for PMN and total leukocyte count was collected into a heparin anticoagulant tube and analyzed within 4 h of extraction. Differential cell count and cytology were examined with a conventional optical microscope. A manual cell count with differential study was done in all samples by experienced technicians.
Ascitic fluid cultures were performed by inoculation of at least 10 mL fluid in VersaTrek® REDOX 1® (TREK Diagnostic Systems, Cleveland, OH). All bottles were processed in a standard fashion according to the manufacturer’s guideline.
SBP was defined as PMN cell count in ascitic fluid ≥ 250/mm3. The 1+ or 2+ result of the leukocyte esterase from dipstick was considered as a positive test. The negative result from dipstick was considered as a negative test. We also defined bacterascites as the combination of a positive ascitic fluid culture, PMN cell count in ascitic fluid < 250/mm3, and no evident intra-abdominal surgically treatable source of infection. In patients with hemorrhagic ascites (i.e. ascites red blood cell count > 10 000/mm3), a subtraction of one PMN leukocyte per 250 red blood cells was made[6]. Secondary peritonitis was suspected when there was an abdominal source of infection, acute pancreatitis, or more than one organism in the ascitic fluid culture.
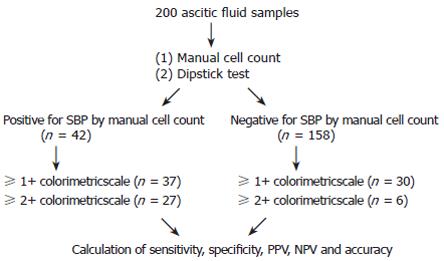
Results of dipstick test were compared to PMN cell count, ascitic fluid culture and clinical data in all patients. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy of dipstick in the diagnosis of SBP from two different colorimetric scales (1+ and 2+) were calculated and compared (Figure 1).
We diagnosed 42 episodes of SBP by PMN cell count, of which 37 (88.1%) was diagnosed using 1+ cut off scale and 27 (64.3%) 2+ cut off scale. Of the 158 specimens with PMN cell count < 250/mm3, 128 had a true negative result by dipstick test (Table 2). Five patients were diagnosed as SBP by manual cell count but had a negative dipstick test result.
| Colorimetric scales | PMN cell count≥250/mm3 | PMN cell count< 250/mm3 | Total |
| 1+ | 10 | 24 | 34 |
| 2+ | 19 | 6 | 25 |
| 3+ | 8 | 0 | 8 |
| 0 | 5 | 128 | 133 |
| Total | 42 | 158 | 200 |
Sixteen specimens had a positive culture for bacteria, of which four were compatible with bacterascites. The dipstick test results in these bacterascites specimens were all negative. No secondary bacterial peritonitis was diagnosed in this series. The most common organism growing from these specimens was E. coli (Table 3).
| Patient | PMN cells/mL | Calorimetric scale | Culture result |
| 1 | 14 | 0 | A.Baumanii |
| 2 | 20 | 0 | S.agalactae |
| 3 | 21 | 0 | P.aeruginosa |
| 4 | 100 | 0 | K.pneumonia |
| 5 | 254 | 0 | E. coli |
| 6 | 271 | 0 | S.bovis |
| 7 | 257 | 0 | K.pneumoniae |
| 8 | 672 | 1 | E. coli |
| 9 | 3640 | 1 | P.aeruginosa |
| 10 | 13 312 | 1 | E. coli |
| 11 | 2129 | 2 | Streptococcus gr.D |
| 12 | 3494 | 2 | Streptococcus gr.D |
| 13 | 12 840 | 2 | P.aeruginosa |
| 14 | 25 805 | 2 | E. coli |
| 15 | 41 990 | 2 | E. coli |
| 16 | 3398 | 3 | E. coli |
The sensitivity, specificity, PPV, NPV and accuracy of 1+ and 2+ cut off scales to diagnose SBP were 88%, 81%, 55%, 96% and 83% respectively, and 63%, 96%, 82%, 91% and 89% respectively (Table 4).
| Variables | ≥1 grade, % | ≥2 grade, % |
| Sensitivity | 88 | 63 |
| Specificity | 81 | 96 |
| Positive predictive value | 55 | 82 |
| Negative predictive value | 96 | 91 |
| Accuracy | 83 | 89 |
Due to the high rates of morbidity and mortality from delayed treatment in cirrhotic patients who suffered from SBP, diagnostic tests for early diagnosis are very important[20,21]. Currently, ascitic fluid PMN count is the standard tool for SBP treatment decision making. However, several-hour delay in reporting the result may arise from the laboratory department. In addition, this test may not be available in a small out patient setting.
Leukocyte esterase has been shown as an important marker for PMN cell activity[22]. Originally, the purpose of leukocyte esterase test was limited to urine analysis[23]. Recently, it has been found to be useful across the wide range of body fluid infections[9-11]. The efficacy of this test in detecting infection in the ascitic fluid has been confirmed from many centers[14-19]. The overall sensitivity, specificity, PPV and NPV of this test are approaching 90% (Table 5)[14-19]. However, the different commercial dipsticks used in previous studies had different colorimetric scales for PMN cell count. In addition, the cut off colorimetric scale for each dipstick has not been standardized yet.
| Authors | Castellote et al | Kim et al | Butani et al | Sapey et al | Thévenot et al |
| Dipsticks | Aution sticks | UriSCAN/Mutistix10SG | Multistix®10SG | Nephur test/ Multistix10SG | Multistix 8SG/Combur2test LN (combined result) |
| Inclusion scale | ≥ 3 (PMN ≥ 250/mm3) | ≥ 3 (PMN ≥ 500/mm3) /≥ 3 (PMN ≥ 75/mm3) | ≥ 2 (PMN ≥ 70/mL mm3) | N/A | ≥ 3 (PMN ≥ 125/mm3) /≥ 2 (PMN ≥ 75/mm3) |
| Sensitivity (%) | 89 | 67/50 | 89 | 88/65 | 89 |
| Specificity( %) | 99 | 100/100 | 99 | 100/100 | 100 |
| PPV (%) | 98 | 100/100 | 89 | 94/92 | 100 |
| NPV (%) | 97 | 89/87 | 99 | 99/97 | 99 |
To date, there has been no dipstick that was specifically designed to use in ascitic fluid. Moreover, many dipsticks do not have a precise colorimetric scale for 250 PMN cells/mm3. In our study, we used the Combur10 Test®M that is widely available in Thailand as a screening test for SBP. According to the manufacturer’s guideline, the 1+, 2+ and 3+ scales are equivalent to 10-25 PMN cells/mm3, 75 PMN cells/mm3 and ≥ 500 PMN cells/mm3 respectively. We chose the lower scales (1+ and 2+) as cut off levels for SBP diagnosis. The limitation of the Combur10 Test®M is an absence of precise calorimetric scale for the cut off level of PMN cell count at ≥ 250/mm3. Because of this limitation, we speculated that our specificity might be lower than others[17-19]. Although our sensitivity (88%) from 1+ cut off level was comparable to others, the specificity and PPV were lower (81% and 55% respectively) compared to other studies[17-19]. By using 2+ scale as a cut off level for SBP, our specificity and PPV increased (96% and 82% respectively), unfortunately the sensitivity decreased to only 63%. Anyhow, NPV and accuracy from these two cut off levels were comparable to other series[17-19].
Aution stick (A. Menarini Diagnostics, Firenze, Italy) is another dipstick that can be read at 90 s. The benefit of the Aution stick over the Combur10 Test®M is the precise colorimetric scale that correlates with ≥ 250 PMN cells/mm3. The correlation between PMN cell count and colorimetric scale suggested by the manufacturer are as follow: grade 0, 0 PMN cell/ mm3; grade 1, 25 PMN cells/ mm3; grade 2, 75 PMN cells/ mm3; grade 3, 250 PMN cells/mm3; and grade 4, 500 PMN cells/mm3. Catellote et al[15] studied the use of Aution sticks for diagnosis of SBP in cirrhotic patients with ascites who underwent abdominal paracentesis at a university based hospital, and found that the sensitivity, specificity and PPV are 89%, 99% and 98%, respectively[15].
Multistix®10SG (Bayer Diagnostics Corporation, Puteaux, France) has colorimetric scales as follow: grade 0, 0 PMN cell/ mm3; grade 1, 15 PMN cells/mm3; grade 2, 70 PMN cells/ mm3; grade 3, 125 PMN cells/ mm3; and grade 4, 500 PMN cells/mm3. Butani et al[24] used the Multistix®10SG to diagnose SBP in 136 specimens by using grade 2 as a cut off scale, and found the sensitivity, specificity, PPV and NPV of the Multistix®10SG are 83%, 99%, 91% and 98% respectively. Although, the Multistix®10SG has no precise colorimetric scale for 250 PMN cells/ mm3, its specificity was still better than that of the Combur10 Test®M in our series. This may be due to a closer 250 PMN/mm3 colorimetric scale of Multistix®10SG (grade 3+ ≥ 125 PMN cells/mm3).
There are many factors that influence the accuracy of the dipsticks. False positive result can result from alteration of pH, osmolality and temperature of the specimens[25]. In addition, non-leukocyte cells can release esterase and produce a false positive result[26]. Moreover, antibiotics can produce both false positive and negative results[27].The false negative result in the present series was found in 5 patients (12%), which is similar to the series presented by Sapey et al[19] None of our patients with false negative result received antibiotics prior to abdominal paracentesis. Interestingly, 3 specimens had a manual PMN cell count between 250/mm3 and 300/mm3 (data not presented), suggesting that the smaller number of PMN cells in these specimens may lead to a false negative result. Campillo et al[28] demonstrated that the sensitivity of dipsticks remains low with PMN cell count ≤ 1000/mm3 when they used Multistix 8 SG and Combur 2 LN as dipstick tests.The false positive rate in our series was 19% which is higher than that of Sapey et al[19], who found that only one patient form each test (Nephur-test® and MutistixSG®) had a false positive result. The possible explanation for a better result from their series is a much closer 250 PMN cells/mm3 of their dipstick scales. Again this information helps to confirm that a precise colorimetric scale for 250 PMN/mm3 is essential for an ideal dipstick to detect SBP.
Currently, there is no specific dipstick test for bacterial count in the ascitic fluid. In our series, it is not surprised that the correlation between the culture and dipstick results was very poor. There were 4 specimens that were compatible with a condition generally called “monomicrobial non-neutrocytic bacterascites (MNB)”. All the MNB specimens had a negative dipstick result. Generally, the clinical outcome of asymptomatic MNB is much better than that of classic SBP[29]. Our 4 patients with MNB did not receive any treatment and were asymptomatic during 3-6 mo follow-up. In addition, repeat ascitic fluid analysis and culture were performed and reported unremarkable results in these 3 patients.
Patients with ≥ 250 PMN cells/mm3 in the absence of bacterial growth from the culture are classified as culture-negative neutrocytic ascites (CNNA). In the present series, only 12 patients (28.6%) had a classic SBP (positive culture and cell count ≥ 250 PMN cells/mm3). Of these culture negative SBP patients, at least 25 (83.3%) had CNNA (no history of prior antibiotics use), demonstrating that CNNA has the same clinical course as classic SBP[6]. Therefore, dipstick that determines mainly for PMN cell activity can be used for SBP screening.
In conclusion, dipstick test in ascitic fluid from cirrhotic patients is a good screening tool for SBP. However, not all dipsticks have a precise colorimetric scale for a measurement of ≥ 250 PMN cells/mm3, therefore the sensitivity of different dipsticks may not be similar. With a good NPV the decision making not to treat SBP based on the results of Combur10 Test®M can be made in the majority of cases. Further study is needed to improve the efficacy of the test in detecting PMN cells and bacteria in ascitic fluid.
The decision making for SBP treatment is mainly based on PMN count. However, manual ascitic fluid PMN count is not always available in every patient care unit.
Leukocyte esterase activity testing by dipstick has been used for a rapid diagnosis of infection in a variety of body fluids such as urine, pleural fluid, and cerebrospinal fluid. The efficacy of this test in detecting infection in the ascitic fluid has been confirmed from many centers. The overall sensitivity, specificity, PPV and NPV of these tests are approaching 90%.
The sensitivity and NPV of dipstick test is excellent in all different types of stick. However, some have a slightly better value due to their approximity to the 250 cell/mm3 cut-off level.
The dipstick may be used as a screening tool for SBP. A better designed test is awaited.
The study used Combur as a screening test. The sensitivity of this test is slightly poorer than others. However, the other validity scores are comparable. The author may need to continue to explore other dipsticks and compare their results using manual cell count as a gold standard.
S- Editor Wang J L- Editor Wang XL E- Editor Liu WF
| 1. | Jepsen P, Vilstrup H, Møller JK, Sørensen HT. Prognosis of patients with liver cirrhosis and spontaneous bacterial peritonitis. Hepatogastroenterology. 2003;50:2133-2136. [PubMed] |
| 2. | Thanopoulou AC, Koskinas JS, Hadziyannis SJ. Spontaneous bacterial peritonitis (SBP): clinical, laboratory, and prognostic features. A single-center experience. Eur J Intern Med. 2002;13:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Boixeda D, De Luis DA, Aller R, De Argila CM. Spontaneous bacterial peritonitis. Clinical and microbiological study of 233 episodes. J Clin Gastroenterol. 1996;23:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Filik L, Unal S. Clinical and laboratory features of spontaneous bacterial peritonitis. East Afr Med J. 2004;81:474-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Deschênes M, Villeneuve JP. Risk factors for the development of bacterial infections in hospitalized patients with cirrhosis. Am J Gastroenterol. 1999;94:2193-2197. [PubMed] |
| 6. | Rimola A, García-Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, Inadomi JM. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32:142-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 612] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 7. | Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351-1355. [PubMed] |
| 8. | Siersema PD, de Marie S, van Zeijl JH, Bac DJ, Wilson JH. Blood culture bottles are superior to lysis-centrifugation tubes for bacteriological diagnosis of spontaneous bacterial peritonitis. J Clin Microbiol. 1992;30:667-669. [PubMed] |
| 9. | Moosa AA, Quortum HA, Ibrahim MD. Rapid diagnosis of bacterial meningitis with reagent strips. Lancet. 1995;345:1290-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Azoulay E, Fartoukh M, Galliot R, Baud F, Simonneau G, Le Gall JR, Schlemmer B, Chevret S. Rapid diagnosis of infectious pleural effusions by use of reagent strips. Clin Infect Dis. 2000;31:914-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Levy M, Tournot F, Muller C, Carbon C, Yeni P. Evaluation of screening tests for urinary infection in hospital patients. Lancet. 1989;2:384-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Kutter D, Figueiredo G, Klemmer L. Chemical detection of leukocytes in urine by means of a new multiple test strip. J Clin Chem Clin Biochem. 1987;25:91-94. [PubMed] |
| 13. | Wisniewski B, Rautou PE, Al Sirafi Y, Lambare-Narcy B, Drouhin F, Constantini D, Fischer D, Labayle D, Denis J. [Diagnosis of spontaneous ascites infection in patients with cirrhosis: reagent strips]. Presse Med. 2005;34:997-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Vanbiervliet G, Rakotoarisoa C, Filippi J, Guérin O, Calle G, Hastier P, Mariné-Barjoan E, Schneider S, Piche T, Broussard JF. Diagnostic accuracy of a rapid urine-screening test (Multistix8SG) in cirrhotic patients with spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol. 2002;14:1257-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Castellote J, López C, Gornals J, Tremosa G, Fariña ER, Baliellas C, Domingo A, Xiol X. Rapid diagnosis of spontaneous bacterial peritonitis by use of reagent strips. Hepatology. 2003;37:893-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Sapey T, Mena E, Fort E, Laurin C, Kabissa D, Runyon BA, Mendler MH. Rapid diagnosis of spontaneous bacterial peritonitis with leukocyte esterase reagent strips in a European and in an American center. J Gastroenterol Hepatol. 2005;20:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Thévenot T, Cadranel JF, Nguyen-Khac E, Tilmant L, Tiry C, Welty S, Merzoug N. Diagnosis of spontaneous bacterial peritonitis in cirrhotic patients by use of two reagent strips. Eur J Gastroenterol Hepatol. 2004;16:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Kim DY, Kim JH, Chon CY, Han KH, Ahn SH, Kim JK, Paik YH, Lee KS, Moon YM. Usefulness of urine strip test in the rapid diagnosis of spontaneous bacterial peritonitis. Liver Int. 2005;25:1197-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Sapey T, Kabissa D, Fort E, Laurin C, Mendler MH. Instant diagnosis of spontaneous bacterial peritonitis using leukocyte esterase reagent strips: Nephur-Test vs. MultistixSG. Liver Int. 2005;25:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Garcia-Tsao G. Current management of the complications of cirrhosis and portal hypertension: variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Gastroenterology. 2001;120:726-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 284] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 21. | Runyon BA, McHutchison JG, Antillon MR, Akriviadis EA, Montano AA. Short-course versus long-course antibiotic treatment of spontaneous bacterial peritonitis. A randomized controlled study of 100 patients. Gastroenterology. 1991;100:1737-1742. [PubMed] |
| 22. | Jacobs JA, De Brauwer EI, Cornelissen EI, Drent M. Correlation of leukocyte esterase detection by reagent strips and the presence of neutrophils: a study in BAL fluid. Chest. 2000;118:1450-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Hiscoke C, Yoxall H, Greig D, Lightfoot NF. Validation of a method for the rapid diagnosis of urinary tract infection suitable for use in general practice. Br J Gen Pract. 1990;40:403-405. [PubMed] |
| 24. | Butani RC, Shaffer RT, Szyjkowski RD, Weeks BE, Speights LG, Kadakia SC. Rapid diagnosis of infected ascitic fluid using leukocyte esterase dipstick testing. Am J Gastroenterol. 2004;99:532-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Triger DR, Smith JW. Survival of urinary leucocytes. J Clin Pathol. 1966;19:443-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Yong WH, Southern JF, Pins MR, Warshaw AL, Compton CC, Lewandrowski KB. Cyst fluid NB/70K concentration and leukocyte esterase: two new markers for differentiating pancreatic serous tumors from pseudocysts. Pancreas. 1995;10:342-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Beer JH, Vogt A, Neftel K, Cottagnoud P. False positive results for leucocytes in urine dipstick test with common antibiotics. BMJ. 1996;313:25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Campillo B, Richardet JP, Dupeyron C. Diagnostic value of two reagent strips (Multistix 8 SG and Combur 2 LN) in cirrhotic patients with spontaneous bacterial peritonitis and symptomatic bacterascites. Gastroenterol Clin Biol. 2006;30:446-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Chu CM, Chang KY, Liaw YF. Prevalence and prognostic significance of bacterascites in cirrhosis with ascites. Dig Dis Sci. 1995;40:561-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |