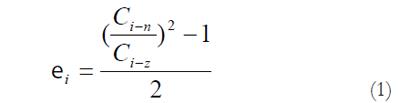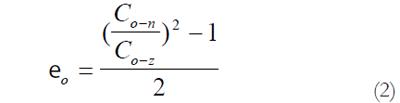Published online Nov 28, 2006. doi: 10.3748/wjg.v12.i44.7149
Revised: July 28, 2006
Accepted: August 15, 2006
Published online: November 28, 2006
AIM: To investigate the effect of a Chinese medicine, Kaiyu Qingwei Jianji (KYQWJJ) used for diabetic treatment, on the morphometry and residual strain distribution of the small intestine in streptozotocin (STZ) -induced diabetic rats. Correlation analysis was also performed between the opening angle and residual strain with the blood glucose level.
METHODS: Forty-two male Wistar rats weighing 220-240 g were included in this study. Thirty-two STZ-induced diabetic rats were subdivided into four groups (n = 8 in each group), i.e. diabetic control group (DM); high dose of KYQWJJ (T1, 36g/kg per day); low dose of KYQWJJ (T2, 17 g/kg per day) and Gliclazide (T3, 50 mg/kg per day). Another ten rats were used as non-diabetic control (CON). The medicines were poured directly into stomach lumen by gastric lavage twice daily. The rats of CON and DM groups were only poured the physiological saline. Blood glucose and plasma insulin levels were measured. Experimental period was 35 d. At the end of experiment, three 5-cm long segments were harvested from the duodenum, jejunum and ileum. Three rings of 1-2 mm in length for no-load and zero-stress state tests were cut from the middle of different segments. The morphometric data, such as the circumferential length, the wall thickness and the opening angle were measured from the digitized images of intestinal segments in the no-load state and zero-stress state. The residual strain was computed from the morphometry data. Furthermore, the linear regression analysis was performed between blood glucose level with morphometric and biomechanical data in the different intestinal segments.
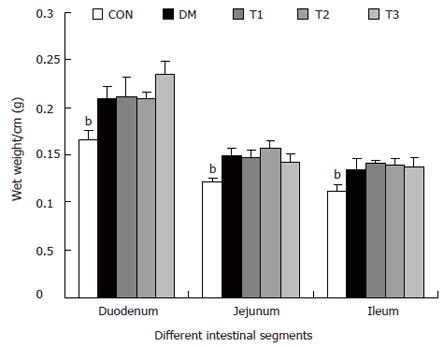
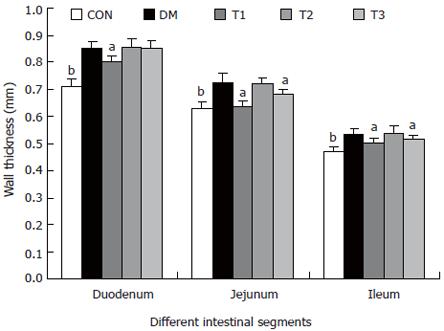
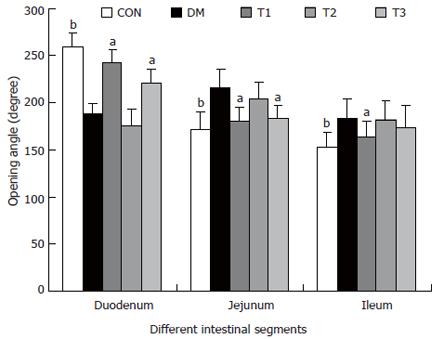
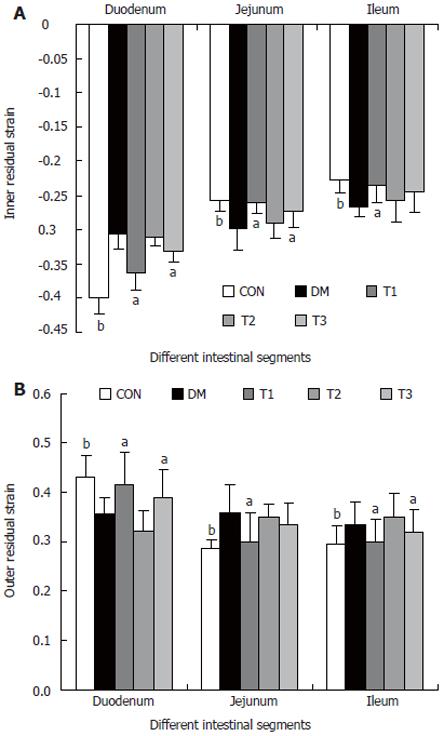
RESULTS: The blood glucose level of DM group was consistent 4-fold to 5-fold higher than those in CON group during the experiment (16.89 ± 1.11 vs 3.44 ± 0.15 mmol/L, P < 0.001). The blood glucose level in the T1 (16.89 ± 1.11 vs 11.08 ± 2.67 mmol/L, P < 0.01) and T3 groups (16.89 ± 1.11 vs 13.54 ± 1.73 mmol/L, P < 0.05), but not in T2 group (P > 0.05) was significantly lower than those in DM group. The plasma insulin levels of DM, T1, T2 and T3 groups were significantly lower than those in CON group (10.98 ± 1.02, 12.52 ± 1.42,13.54 ± 1.56,10.96 ± 0.96 vs 17.84 ± 2.34 pmol/L respectively, P < 0.05), but no significantly difference among the groups with exception of CON group. The wet weight/cm and total wall thickness of duodenum, jejunum and ileum in DM group were significantly higher than those in CON group (wet weight (g/cm): duodenum 0.209 ± 0.012 vs 0.166 ± 0.010, jejunum 0.149 ± 0.008 vs 0.121 ± 0.004, ileum 0.134 ± 0.013 vs 0.112 ± 0.007; Wall thickness (mm): duodenum 0.849 ± 0.027 vs 0.710 ± 0.026, jejunum 0.7259 ± 0.034 vs 0.627 ± 0.025, ileum 0.532 ± 0.023 vs 0.470 ± 0.010, all P < 0.05), T1 and T3 treatment could partly restore change of wall thickness, but T2 could not. The opening angle and absolute value of inner and outer residual stain were significantly smaller in duodenal segment (188 ± 11 degrees, -0.31 ± 0.02 and 0.35 ± 0.03 vs 259 ± 15 degrees, -0.40 ± 0.02 and 0.43 ± 0.05) and larger in jejunal (215 ± 20 degrees, -0.30 ± 0.03 and 0.36 ± 0.06 vs 172 ± 19 degrees, -0.25 ± 0.02 and 0.27 ± 0.02) and ileal segments (183 ± 20 degrees, -0.28 ± 0.01 and 0.34 ± 0.05 vs 153 ± 14 degrees, -0.23 ± 0.03 and 0.29 ± 0.04) in DM group than in CON group (P < 0.01). T1 and T3 treatment could partly restore this biomechanical alteration, but strong effect was found in T1 treatment (duodenum 243 ± 14 degrees, -0.36 ± 0.02 and 0.42 ± 0.06, jejunum 180 ± 15 degrees, -0.26 ± 0.03 and 0.30 ± 0.06 and ileum 163 ± 17 degrees, -0.23 ± 0.03 and 0.30 ± 0.05, compared with DM, P < 0.05). The linear association was found between the glucose level with most morphometric and biomechanical data.
CONCLUSION: KYQWJJ (high dose) treatment could partly restore the changes of blood glucose level and the remodeling of morphometry and residual strain of small intestine in diabetic rats. The linear regression analysis demonstrated that the effect of KYQWJJ on intestinal opening angle and residual strain is partially through its effect on the blood glucose level.
- Citation: Sha H, Zhao JB, Zhang ZY, Zhou SP, Tong XL, Zhuang FY, Gregersen H. Effect of Kaiyu Qingwei Jianji on the morphometry and residual strain distribution of small intestine in experimental diabetic rats. World J Gastroenterol 2006; 12(44): 7149-7154
- URL: https://www.wjgnet.com/1007-9327/full/v12/i44/7149.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i44.7149
Dysfunction of upper gastrointestinal tract (GI) is common among diabetic patients[1]. As many as 75% of patients visiting diabetes clinics report significant GI Symptoms[2,3]. Common complaints include dysphagia, early satiety, reflux, abdominal pain, nausea, vomiting, and diarrhea[2,3]. As with other complications of diabetes, the duration of the disorder and poor glycemic control seem to be associated with more severe GI problems[2,3]. Histologically, many experiments have demonstrated prominent proliferation of GI wall layers, especially mucosa in the small intestine and esophagus of diabetes[4-9]. Many studies have shown that DM causes morphological changes and biomechanical remodeling in the esophagus[10,11], stomach[12] and small intestine[13,14]. Recently, Frøkjær et al in a human study found an increase in esophageal wall thickness and altered deformation to the distension with reduced longitudinal shortening and the radial stretch in insulin dependent diabetes mellitus (IDDM) patients (unpublished data). The biomechanical remodeling of the GI tract likely plays an important role in the GI disorder of diabetic patients[1]. Therefore, it is important to study the biomechanical properties of the small intestine in diabetic animals. Remodeling of the structural and biomechanical properties can be measured as changes in the residual strain and stress-strain distributions. Residual strain is defined as the strain in the no-load state (where external forces are zero) in reference to the zero-stress state.
To the best of our knowledge, only few studies related to the residual strain in diabetic small intestine have been studied[13,15]. Previously we have demonstrated that gliclazide treatment could partially restore the changes of biomechanical parameters of small intestine in the diabetic rats[15]. Regarding the Kaiyu Qingwei Jianji (KYQWJJ), several previous studies have demonstrated that it could decrease blood glucose level, decrease blood lipid level, increase sensitivity of insulin and decrease resistance of insulin[16-18]. However, no study has been performed about the effect of KYQWJJ on the morphometric and biomechanical properties of small intestine in diabetes. Therefore, the goal of the present study was to investigate the effect of KYQWJJ on the remodeling of the zero-stress state of the small intestine. The gliclazide is serving as positive control in the present study.
Fifty male Wistar rats weighing 220-240 g were included in this study. Forty rats were made diabetic by a single intraperitoneal injection of 60 mg/kg streptozotocin (STZ, Sigma Company). This dose resulted in a fasting serum glucose level greater than 11.1 mmoL/L in 80% of rats after 4 d of injection; the remaining 20% were finally not used in this study. Another 10 rats of similar age and body weight from the same vendor were used as non-diabetic control (CON). Thirty-two diabetic rats were subdivided into four groups (n = 8 in each group), i.e. diabetes mellitus control group (DM); high dose of KYQWJJ (T1); low dose of KYQWJJ (T2) and Gliclazide (T3).
KYQWJJ was composed of Radix Bupleuri, Radix et Rhizoma Rhei, Rhizoma Pinelliae and Rhizoma Coptidis, and provided by China-Japan Friendship Hospital. Gliclazide was purchased from Hua Ju pharmaceutical factory (Tanjun, China). The medicines were injected directly into stomach lumen by gastric lavage twice daily and the dosage was 36 g/kg for T1, 17 g/kg for T2 and 50 mg/kg for T3 respectively. The rats of CON and DM groups were only poured the physiological saline.
Weight and blood glucose levels were measured 5 d, three weeks and five weeks after initiating the experiment. The plasma insulin level was measured when the experiment finished. Experimental period was 35 d.
After 35 d, the animals were anesthetized with sodium pentobarbital (50 mg/kg ip). Following laparatomy, the calcium antagonist, papaverine (60 mg/kg) was injected into the lower thoracic aorta through a cannula (22 G/25 mm) in order to abolish contractile activity in the GI tract. After obtaining smooth muscle relaxation, the whole small intestine was harvested. The most proximal segment was close to the pylorus whereas the most distal segment was close to the ileo-cacal valve. The length of duodenum and whole jejunum and ileum in vitro was measured. Within a short time, the residual contents in the lumen were gently cleared using saline and the wet weight of duodenum, jejunum and ileum was measured. Three 6 cm-long segments from duodenum, jejunum and ileum were harvested. The duodenum was taken from the descending part, 1 cm down the pylorus; the jejunum from 5 cm distal to the ligament of Treitz, and the ileum from 5 cm proximal to the ileo-cecal valve. Then the segments were placed immediately into cold aerated Krebs solution containing 6% dextran. Three rings, 1-2 mm in length, were cut from the middle part of each segment for no-load and zero-stress state tests. The rings were photographed in the no-load state using a video camera (SONY CCD Camera, Japan) and then cut radially on the anti-mesenteric side to obtain their zero-stress state. A 60-min-period was allowed for equilibration and the specimens were photographed again. The selection of this time period was based on pilot experiments.
The morphometric data were obtained from the digitalized images of the photographs of the segments in the zero-stress and no-load states. Measurements were done using SigmaScan software (Jandel Scientific, Germany). The following data were measured from each specimen: the circumferential length (C), the wall thickness (h), and the opening angle at the zero-stress state. The subscripts i, o, n, and z refer to the inner (mucosal) surface, outer (serosal) surface, no-load state and zero-stress state condition. The data from triplet of rings were averaged before further analysis.
The measured data was used for computation of residual strains defined as:
Residual Green’s strain at the mucosal surface:
Math 1
Residual Green’s strain at the serosal surface:
Math 2
Thus, from the circumferential lengths at the no-load and zero-stress state, we can compute the circumferential residual strain, at the mucosal and serosal surfaces in the sense of Green strain. Green strain is used when large deformations are encountered as in this study. Negative strain implies that the tissue is in compression whereas positive strain implies extension.
The association between the glucose level with opening angle and residual strain in different groups with referenced to the DM group were performed using the linear regression analysis.
The data were representative of a normal distribution and accordingly the results were expressed as mean ± SD unless otherwise stated. Analysis of variance was used to detect difference among the different groups (Sigmastat 2.0TM). Linear regression analysis was used to demonstrate possible association between the blood glucose levels and opening angle and residual strain (Sigmastat 2.0TM). The results were regarded as significant when P < 0.05.
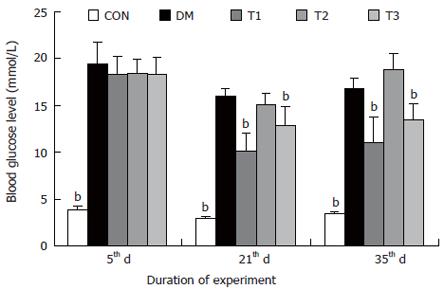
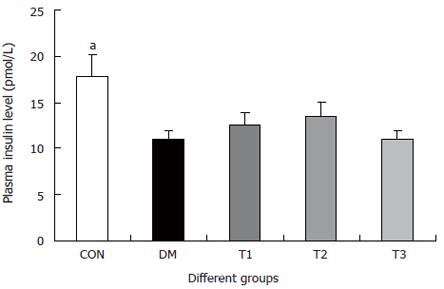
The changes of the blood glucose level during the development of diabetes are shown in Figure 1. The blood glucose level of DM group was consistent 4-fold to 5-fold higher than those in CON group during the experiment (F = 19.4, P < 0.001). Compared with DM group, significant lower of blood glucose level was found in the T1 and T3 groups (F = 16.1, P < 0.01 and F = 5.8, P < 0.05), but not in T2 group (F = 0.8, P > 0.05). The plasma insulin level was shown in Figure 2. The plasma insulin levels of DM, T1, T2 and T3 groups were significantly lower than those in CON group (P < 0.05), but no significantly difference was found among the groups with exception of CON group (P > 0.05).
The wet weight per centimeter and the wall thickness were increased during diabetes in the duodenum, jejunum and ileum (P < 0.01). After treatment with T1, T2 and T3, the wet weight per centimeter of different segments did not change significantly. However, T1 and T3 partly inhibited the wall thickness increase, in which the effect of T1 was stronger (P < 0.05).
The opening angles and residual strains of the different intestinal segments were shown in Figures 5 and 6. In brief, the opening angles and absolute value of residual strain were smaller in duodenum and larger in jejunum and ileum in the DM group when compared to those in CON group (P < 0.01). T1 and T3 treatment could partly restore this biomechanical alteration, in which the effect of T1 was stronger (P < 0.05). No significant effect in T2 treatment was found.
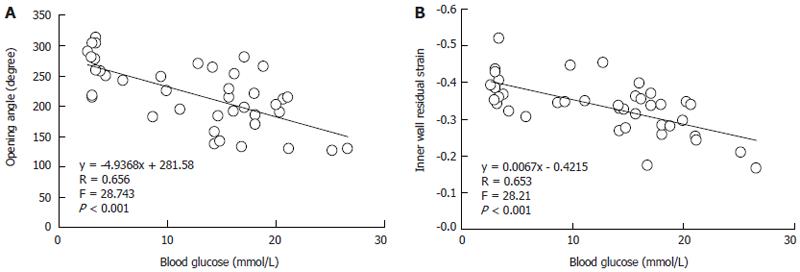
Linear regression analysis indicated that the linear association existed between blood glucose level with most morphometric and biomechanical data (Table 1). The examples of association between the glucose with opening angle and inner residual strain in duodenal segment were shown in the Figure 7. The high linear association existed between blood glucose level with opening angle (r = 0.657, P < 0.001, Figure 7A) and inner residual strain (r = 0.653, P < 0.001, Figure 7B).
| Duodenum | Jejunum | Ileum | |||||||
| F | P | F | P | F | P | ||||
| Opening angle | y = -4.937x + 281.6 | 28.7 | < 0.001 | y = 4.253x + 131.7 | 20.4 | < 0.001 | y = 2.244x + 142.6 | 4.7 | 0.038 |
| R = 0.656 | R = 0.591 | R = 0.33 | |||||||
| Inner residual strain | y = 0.007x - 0.422 | 28.2 | < 0.001 | y = -0.003x - 0.236 | 9.5 | 0.004 | y = 0.001x - 0.286 | 0.7 | 0.418 |
| R = 0.653 | R = 0.447 | R = 0.13 | |||||||
| No-load wall thickness | y = 0.007x + 0.707 | 18.3 | < 0.001 | y = 0.004x + 0.617 | 5.9 | 0.02 | y = 0.003x + 0.485 | 4.4 | 0.042 |
| R = 0.575 | R = 0.37 | R = 0.32 | |||||||
Studies of the gut in the streptozotocin model of diabetes have almost entirely involved use of the rat. The form of diabetes mellitus induced by parenteral streptozotocin administration in rats is insulin-dependent (type I). Without treatment with insulin, STZ-induced diabetic rats become hyperglycaemic, polyphagic, polidipsic, poliuric and undernourished. In our studies, the blood glucose level was 4-5 times higher in diabetic rats than in non-diabetic rats. The major findings in our studies were that the opening angle and residual strain were smaller in the duodenum and larger in the jejunum and ileum in diabetic rats compared to normal rats. After gliclazide and high dose KYQWJJ treatment, these biomechanical parameters trend to back to normal state. Furthermore, we confirmed previous data that the intestinal weight per-unit length prominently increased in the diabetic rats[9,12-14], gliclazide and high dose KYQWJJ treatment can partly reverse the remodeling of intestine. High dose KYQWJJ seems better than gliclazide to reverse the morphometric and biomechanical remodeling of intestine in STZ -induced diabetic rats.
The small intestine is the longest section of the digestive tract and consists of three segments, namely the duodenum, jejunum and ileum, forming a passage from the pylorus to the large intestine. The main functions of the small intestine can be roughly divided into transport, absorption and secretion. The movement of luminal contents is facilitated by changes in the geometry of the wall and lumen due to the motility. It is well known that a bolus propelled in front of a peristaltic contraction distends the gut wall[19]. Therefore, it is important to know the passive mechanical wall properties devoid of any neurohumoral regulatory mechanisms. The biomechanical properties of the small intestine depend on its structure. Because significant structural remodeling of the intestine is occurred in the diabetes[3-9,12], therefore, the biomechanical properties of the small intestine may be also changed. To our understanding of knowledge, we consider the zero-stress state and residual strain as the most relevant quantitative aspect of remodeling because it is a measure of the non-uniformity of growth or resorption in different parts of the intestinal wall. Furthermore, the structural components of the intestinal wall must be measured at the zero-stress state because in this state the morphology and sizes of the cells and extracellular matrix are not distorted by stress and strain. In our present experiment, during the development of diabetes, the opening angles and absolute value of residual strains were decreased in the duodenum and increased in the jejunum and ileum when compared to those in the controls. High dose KYQWJJ and Gliclazide treatment partly restored this biomechanical alteration, the effect of the former is stronger. In the previous study we have discussed the possible mechanism of effect of Gliclazide on the diabetic intestinal remodeling[15]. Regarding the KYQWJJ, several previous studies have demonstrated that it could decrease blood glucose level, decrease blood lipid level, increase sensitivity of insulin and decrease resistance of insulin[16-18]. We confirmed in the present study that KYQWJJ could decrease blood glucose level in the diabetic rats. The linear regression analysis demonstrated that the linear association existed between blood glucose level with most morphometric and biomechanical data. Therefore, the effect of KYQWJJ on intestinal opening angle and residual strain is partially through its effect on the blood glucose level. These seem to be the part mechanism for the effect of KYQWJJ on the diabetic intestinal remodeling. However, regarding more detail mechanisms of effect of KYQWJJ on the diabetic intestinal remodeling, further studies should be done.
The changes in the opening angle of intestine during the development of diabetes shown in this study suggest that the morphological and biomechanical remodeling of different layers are an important determinant of growth and remodeling of the zero-stress state. Fung’s hypothesis of non-uniform remodeling suggested that if the inner wall grows more than the outer wall, the opening angle will increase; whereas if the outer wall grows more than the inner wall, the opening angle will decrease[20]. In this experiment, since the changes in jejunum and ileum primarily are in the mucosa layers, the inner wall grows more than the outer wall and hence the opening angle increased. Correspondingly, the outer residual strain became more tensile whereas the inner residual strain became more compressive in these two segments. But in the duodenal segment, the opening angle and absolute value of residual strain were decreased during the diabetes. We have demonstrated that the all layers of duodenum increased after experimental diabetes and the wall became stiffer[13], at this condition the biomechanical properties of layers may determine the remodeling of the zero-stress state. Because the wall becomes stiffer, the opening angle may become smaller.
The GI wall structure or deformation changes in the diabetes may alter the relative positions of the mechano-sensitive afferents (zero setting of the mechanosensitive afferents)[21]. The biomechanical remo-deling in the diabetes such as alterations of residual strain and stress distribution[15] and increase the wall stiffness[13,14] will alter the tension and stress distribution of the mechanosensitive afferents[22,23]. As results, the perception and motility of the GI tract will change as well. Hence, the morphological changes and biomechanical remodeling of GI tract in the diabetes is likely to affect the function of mechanosensitive afferents in the GI wall and further affect the motor and sensory function. After KYQWJJ treatment, the structural remodeling of different segments was partly improved, accordingly the biomechanical remodeling of these segments was partly reversed. Therefore, the KYQWJJ should have the effect to improve motor and sensory dysfunction of diabetic small intestine.
In conclusion, Chinese medicine of KYQWJJ treat-ment could partly restore the changes of blood glucose and insulin levels and remodeling of morphometry and residual strain of small intestine in STZ-induced diabetic rats. The linear regression analysis demonstrated that the effect of KYQWJJ on intestinal opening angle and residual strain is partially through its effect on the blood glucose level. Therefore, it is possible to develop some Chinese herbs, such as KYQWJJ, to improve the intestinal dysfunction and further may be applicable in the clinics.
S- Editor Wang GP L- Editor Lutze M E- Editor Ma WH
| 1. | Zhao J, Frøkjaer JB, Drewes AM, Ejskjaer N. Upper gastrointestinal sensory-motor dysfunction in diabetes mellitus. World J Gastroenterol. 2006;12:2846-2857. [PubMed] |
| 2. | Folwaczny C, Riepl R, Tschöp M, Landgraf R. Gastrointestinal involvement in patients with diabetes mellitus: Part I (first of two parts). Epidemiology, pathophysiology, clinical findings. Z Gastroenterol. 1999;37:803-815. [PubMed] |
| 3. | Verne GN, Sninsky CA. Diabetes and the gastrointestinal tract. Gastroenterol Clin North Am. 1998;27:861-74, vi-vii. [PubMed] |
| 4. | Zoubi SA, Williams MD, Mayhew TM, Sparrow RA. Number and ultrastructure of epithelial cells in crypts and villi along the streptozotocin-diabetic small intestine: a quantitative study on the effects of insulin and aldose reductase inhibition. Virchows Arch. 1995;427:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Mayhew TM, Carson FL, Sharma AK. Small intestinal morphology in experimental diabetic rats: a stereological study on the effects of an aldose reductase inhibitor (ponalrestat) given with or without conventional insulin therapy. Diabetologia. 1989;32:649-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Zoubi SA, Mayhew TM, Sparrow RA. The small intestine in experimental diabetes: cellular adaptation in crypts and villi at different longitudinal sites. Virchows Arch. 1995;426:501-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Tahara T, Yamamoto T. Morphological changes of the villous microvascular architecture and intestinal growth in rats with streptozotocin-induced diabetes. Virchows Arch A Pathol Anat Histopathol. 1988;413:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Charlton M, Ahlman B, Nair KS. The effect of insulin on human small intestinal mucosal protein synthesis. Gastroenterology. 2000;118:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Jørgensen CS, Ahrensberg JM, Gregersen H, Flyvberg A. Tension-strain relations and morphometry of rat small intestine in experimental diabetes. Dig Dis Sci. 2001;46:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Yang J, Zhao J, Zeng Y, Gregersen H. Biomechanical properties of the rat oesophagus in experimental type-1 diabetes. Neurogastroenterol Motil. 2004;16:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Zhao J, Liao D, Gregersen H. Biomechanical and histomorphometric esophageal remodeling in type 2 diabetic GK rats. J Diabetes Complications. 2006;21:34-40. [PubMed] |
| 12. | Liao D, Zhao J, Gregersen H. Three-dimensional geometry analysis of the stomach in type II diabetic GK rats. Diabetes Res Clin Pract. 2006;71:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Zhao J, Yang J, Gregersen H. Biomechanical and morphometric intestinal remodelling during experimental diabetes in rats. Diabetologia. 2003;46:1688-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Zhao J, Liao D, Yang J, Gregersen H. Viscoelastic behavior of small intestine in streptozotocin-induced diabetic rats. Dig Dis Sci. 2003;48:2271-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Zhao J, Sha H, Zhou S, Tong X, Zhuang FY, Gregersen H. Remodelling of zero-stress state of small intestine in streptozotocin-induced diabetic rats. Effect of gliclazide. Dig Liver Dis. 2002;34:707-716. [PubMed] |
| 16. | Po XY, Tong X. The effect of Ke Yu Qing Wei Ke li on sensitivity of insulin in Type 2 diabetes. Zhongguo Zhongyiyao Xinxi Zazhi. 2003;10:11-13. |
| 17. | Tong Xl, Liu HF, Po XY, Zhong SP. Clinical study about treatment of Ke Yu Qing Wei Ke lion resistance of insulin in Type 2 diabetes. Shiyong Zhongyineike Zazhi. 2001;15:10-11. |
| 18. | Liu HF, Tong XL, Wang QG, Zuo PP, Guo AC, Liu HX. The effect of Ke Yu Qing Wei Ke li on insulin receptor of liver and skeletal muscle in rats. Beijing Zhaongyiyao Daxue Xuebao. 2002;25:35-37. |
| 19. | Siegle ML, Ehrlein HJ. Effects of various agents on ileal postprandial motor patterns and transit of chyme in dogs. Am J Physiol. 1989;257:G698-G703. [PubMed] |
| 20. | Fung YC. What are the residual stresses doing in our blood vessels. Ann Biomed Eng. 1991;19:237-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 192] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Pedersen J, Gao C, Egekvist H, Bjerring P, Arendt-Nielsen L, Gregersen H, Drewes AM. Pain and biomechanical responses to distention of the duodenum in patients with systemic sclerosis. Gastroenterology. 2003;124:1230-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Raab M, Neuhuber WL. Number and distribution of intraganglionic laminar endings in the mouse esophagus as demonstrated with two different immunohistochemical markers. J Histochem Cytochem. 2005;53:1023-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Swithers SE, Baronowsky E, Powley TL. Vagal intraganglionic laminar endings and intramuscular arrays mature at different rates in pre-weanling rat stomach. Auton Neurosci. 2002;102:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |