Published online Nov 28, 2006. doi: 10.3748/wjg.v12.i44.7136
Revised: June 28, 2006
Accepted: September 1, 2006
Published online: November 28, 2006
AIM: To evaluate the antibacterial activity of Sapindus mukorossi (S. mukorossi) and Rheum emodi (R. emodi).
METHODS: Powders of S. mukorossi and R. emodi were extracted successively with petroleum ether, benzene, chloroform and ethanol and were concentrated in vacuum. The disk diffusion method was used for in vitro studies and in vivo studies were performed on male Wister rats. Thirty resistant clinical isolates of H pylori, as determined by their antibiotic sensitivity patterns by E-test, along with two Gram +ve (S. aureus, B. subtilis) and two Gram -ve (E. coli, P. vugaris) organisms were screened for their susceptibility patterns against these extracts.
RESULTS: In our screening, all 30 resistant isolates and the other four organisms (two Gram +ve S. aureus, B. subtilis and two Gram -ve, E. coli, P. vugaris) were sensitive to the test compounds. It was found that ethanol and chloroform extracts of S. mukorossi and ethanol and benzene extracts of R. emodi inhibited H pylori at very low concentrations. In the in vitro study, the isolates showed a considerable zone of inhibition at very low concentrations (10 μg/mL) for both the extracts. In the in vivo study, the H pylori infection was cleared with minimal doses of extracts of S. mukorossi (2.5 mg/mL) and R. emodi (3.0 mg/mL) given orally for seven days.
CONCLUSION: We can conclude from this study that the extracts of S. mukorossi and R. emodi inhibited the growth of pylori in vitro and, in in vivo studies, the H pylori infection cleared within seven days at very low concentrations. We also found that H pylori did not acquire resistance against these herbal extracts even after 10 consecutive passages.
-
Citation: Ibrahim M, Khan AA, Tiwari SK, Habeeb MA, Khaja M, Habibullah C. Antimicrobial activity of
Sapindus mukorossi andRheum emodi extracts againstH pylori :In vitro andin vivo studies. World J Gastroenterol 2006; 12(44): 7136-7142 - URL: https://www.wjgnet.com/1007-9327/full/v12/i44/7136.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i44.7136
H pylori has infected more than half the population of the world. Most people are unaware that they are infected because they remain asymptomatic throughout life and survive without any harmful infection-related clinical sequelae. However, some develop duodenal or gastric ulcers and a small proportion are diagnosed with MALT lymphoma or gastric malignancy[1]. Others suffer from non-specific dyspeptic symptoms with no obvious cause other than H pylori infection and its associated gastritis.
Global elimination of H pylori is a noble goal that would have a major impact on present and future world health. During the past decade effective treatment therapies have been developed to cure H pylori infection, following the work of Bazzoli et al[2] and Lamouliatte et al[3]. Current treatment is based on a combination of a proton pump inhibitor (PPI) with either amoxycillin or metronidazole. Large multicentric studies, especially MACH 1[4] and MACH 2[5], have confirmed the excellent results of pilot studies leading to eradication rates higher than 90%. Unfortunately, in subsequent years such good results have not been achieved, especially in South East Asia and Southern Europe, where resistance to antibiotics has become more prevalent. Complete eradication of H pylori is still in the initial stage. Resistance to amoxycillin, clarithromycin and metronidazole is widespread, and probably increasing as a result of the constant use of these drugs against H pylori infection. In our recent multicentric study, undertaken by the Indian H pylori study group, we assessed the Indian scenario of resistance[6]. The study showed higher resistance to antibiotics than in developed countries[7]. In this study, 100% resistance of H pylori towards metronidazole was reported in Hyderabad, which was much higher compared to other regions of India. If this trend continues the treatment with clarithromycin and metronidazole may become ineffective.
In view of the incomplete cure achieved with the triple therapy described above and its possible side effects, alternative medicine cures are gaining much prominence and are found to be safe and effective eliminators of H pylori infection. Thus, in an endeavor to overcome increasing resistance, we continued to look for some selected herbal extracts that are capable of inhibiting the growth of H pylori with minimal or no side effects. In the present study, we selected two plants, namely Sapindus mukorossi and Rheum emodi, and investigated the inhibitory effect of these plant extracts against H pylori in vitro and in vivo.
S. mukorossi Gaerten (Sapindaceae), commonly known as Ritha or Aritha is found throughout India. The major constituents of its fruit are saponins (10%-11.5%), sugars (10%) and mucilage[8]. The fruit of the plant is reported to have expectorant, emetic, alexipharmic and abortificiant effects. It is also used for excessive salivation, epilepsy and chlorosis[9,10]. Saponins from this plant are known to be spermicidal in vitro[11]. This spermicidal property has been used in contraceptive cream[12]. The alcoholic extract of (Sapindus trifoliatus Linn) is reported to possess anti-implantation activity[11].
R. emodi (Polygonaceae), commonly known as Indian or Himalayan Rhubarb, is found in India. The major constituents of rhubarb rhizomes are anthraquinones. Rhubarb is used as a laxative and diuretic to treat kidney stones and it is used for gout and liver diseases characterized by jaundice. Externally, it is used to heal skin sores and scabs. Paradoxically, larger doses are used as a laxative, although small doses are used to treat dysenteric diarrhea[13]. The Chinese use rhubarb as an ulcer remedy, anti-helminthic and to treat cancer, upper intestinal bleeding (ulcers), fever, and headache. They consider it to be a bitter, cold dry herb used to “clear heat” from the liver, stomach and blood[14,15]. It is also used to treat toothache[16]. In Europe, rhubarb is a component of spring tonics or blood cleansing cures, including Swedish bitter[17]. Turkish or medicinal rhubarb is also one of the four major ingredients in the herbal cancer remedy.
We isolated extracts from both plants and carried out antimicrobial screening for various micro-organisms. Based on the above results, a study was designed using the obtained products of S. mukorossi and R. emodi to assess the antibacterial activity against H pylori in vitro and in vivo.
Authentic samples of S. mukorossi and R. emodi were obtained from an authorized supplier (M/s Munnalal Dawasas and Co. Hyderabad, Andhra Pradesh, India). The plants were identified and authenticated by experts in the Post Graduate and Research Department of Botany, Anwar-ul-loom College, Hyderabad, Andhra Pradesh, India.
Thirty H pylori strains along with two gram +ve (Staphylococcus aureus, Bacillus subtilis) and two gram -ve (Escerichia coli, Proteus vulgaris) pathogenic bacteria were used in the study. The H pylori strains were isolated from gastric biopsy specimens [15 from duodenal ulcer (DU), 8 from gastric ulcer (GU), 4 from non-ulcer dyspepsia (NUD), 3 from gastric carcinoma (GC)], after informed consents were obtained from patients who underwent upper gastrointestinal endoscopy at Deccan College of Medical Sciences, Hyderabad, India. The other four pathogens were obtained in pure culture form from the Department of Microbiology, Deccan College of Medical Sciences, Hyderabad, A. P, India.
E test strips were obtained from AB Biodisk, Solna, Sweden.
Male Wister rats (175-200 g) were acclimated to the housing facilities for 5 d before initiation of the study. Free access to standard pellet chow was allowed throughout the experimental protocol, with the exception of overnight fasting before induction of the ulcer. All protocols were approved by the Animal Care and Use Committee of the Deccan College of Medical Sciences and Research Centre, Hyderabad, India where the study was conducted.
For phytochemical analysis, approximately 100 g of fruit pericarp of S. mukorossi and rhizomes of R. emodi were collected and the materials were chopped, air dried at 35-40°C and pulverized in an electric grinder. The powder obtained was successively extracted in petroleum ether (60-80°C), benzene, chloroform and ethanol.
The extracts were then powdered by using a rotary evaporator under reduced pressure. Fruit pericarp of S. mukorossi yielded 38g, 28g, 34g and 35g of powdered extracts with petroleum ether, benzene, chloroform and ethanol respectively. Rhizomes of R. emodi yielded 19 g, 17 g, 21 g and 22 g of powdered extracts. Extracts obtained by percolation using 70% of ethanol as a solvent at room temperature were processed according to process A of Farmacopeia dos Eastados Unidos do Brasil (1959) (AOAC 1990).
The extracts were evaporated at 40°C under vacuum and the residue was freeze-dried. The dry extracts of the fruit pericarp of S. mukorossi and rhizomes of R. emodi were tested for the presence of saponins and anthraquinones. Each extract of the fruit pericarp of S. mukorossi (SM) and rhizomes of R. emodi (RE) were column chromatographed over a silica gel (200 mesh), eluted with CHCl3-MeOH (70:30, 60:40, 50:50, 25:75) and compound fractions of (250 mL each) were collected and monitored by TLC. These column chromatographed compound fractions were further filtered to yield saponins and anthraquinones, which were separated by paper chromatography and preparative TLC to yield saponins [(SM-A (petroleum ether), SM-B (benzene), SM-C (chloroform) and SM-D (ethanol)] and anthraquinones [(RE-A (petroleum ether), RE-B (benzene), RE-C (chloroform) and RE-D (ethanol)] respectively.
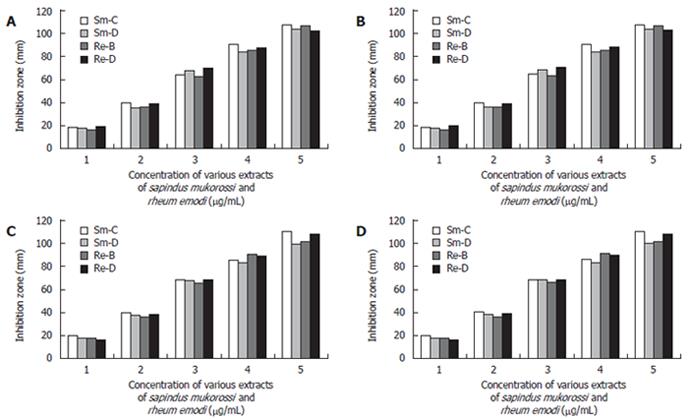
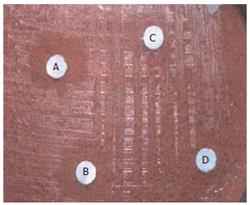
All the filtrates obtained were dried by evaporation (Rotometer, 40°C) and the dried extracts were individually once again dissolved in 10 mL ethanol (95%). Then subjected to a complete drying process and weighed according to the AOAC (1990) method[18]. The products obtained were tested initially for antimicrobial activity against different gram +ve organisms (S.aureus, B.subtilis) and gram -ve organisms (E.coli, P.vulgaris). Effective antibacterial activity was noted (Figure 1A-1D).
From every patient a total of three-biopsy specimens were collected, 1 in urea solution and two biopsies (1 from the antrum and the other from the corpus) were collected in Brucella broth supplemented with 2% fetal calf serum [FCS] (Gibco BRL, Germany) and 10% glycerol. The biopsy specimens were smeared directly onto Brucella agar (Difco Chemicals, Detroit, USA) supplemented with 7% (v/v) sheep blood, Vancomycin- 6 mg/mL, Amphoteracin B- 2mg/mL and Polymixin B- 2500 IU/mL (Sigma Chemicals Inc, Bangalore).
The agar plates were incubated at 37°C for 3-5 d under microaerobic conditions (4% Oxygen, 5% Hydrogen, 5% Carbon-di-oxide, and 86% Nitrogen) in a candle jar dessicator. H pylori were identified based on the culture characteristics, such as small translucent colonies (2-4 mm) under a dissected microscope, gram -ve spiral organisms, positive for catalase, cytochrome oxidase and urease tests. Stocks of pure culture were preserved in Brucella broth (Difco Chemicals, Detroit, USA) supplemented with 10% FCS and 15% glycerol and stored in -80°C and revived when required.
The antibiotic sensitivity patterns of all the 30 H pylori strains towards commonly used antibiotics such as metronidazole, amoxycillin, tetracycline and clarithromycin were evaluated by the E-test method. All the strains were seeded on Brucella agar supplemented with 7% sheep blood and grown for 48 h under microaerobic conditions. Bacterial growth was scraped from the plates and resuspended in sterile saline. The inoculum was prepared to contain 108 CFU/mL by adjusting the suspension to match the McFarland No-0.5 turbidity standard for MIC studies[19] and 2 × 108 CFU/mL of Mc Farland no- 1 standard for antibiotic sensitivity studies[20,21] (Table 1).
| Antibiotic | Sensitiven (%) | Resistancen (%) | Range(μg/mL) | MIC1 (μg/mL) |
| Metronidazole | 7 (23.4) | 23 (76.6) | 0.0125 - > 256 | > 8 |
| Clarithromycin | 28 (93.3) | 2 (6.7) | < 0.016 - > 256 | > 2 |
| Amoxicillin | 28 (93.3) | 2 (6.7) | < 0.016 - > 256 | > 0.5 |
| Tetracycline | 28 (93.3) | 2 (6.7) | < 0.016 - 2 | > 4 |
Growth inhibition was performed by the filter paper disc diffusion method[22] on brucella agar plates supplemented with 7% sheep blood under micro-aerobic conditions at 37°C. To check the antimicrobial activity of the extracts and to read the antibiotic sensitivity of the isolates, inoculum was prepared as described above and the culture was spread over the plates with the help of a sterile cotton swab.
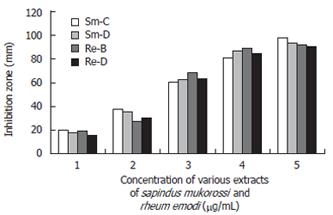
Various concentration disks (10-200 μg/mL) of the plant extracts were prepared and the latter were placed on brucella agar plates with 7% sheep blood earlier inoculated with 50 μL bacterial suspension in brucella broth with 5% FCS (108-109 CFU/mL). This was done to evaluate the minimum concentration of the extracts at which H pylori growth was inhibited effectively. The MIC was determined by measuring the zone of inhibition at each concentration after incubation at 37°C for 3-5 d (Figure 2).
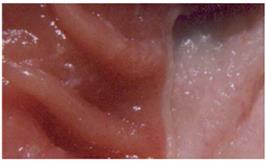
Induction of ulcers: Ulcers were induced using a model modified from that described by Okabe and Pfeiffer[23]. Briefly, rats were given acetic acid (0.5 mL of 80% vol/vol) with a syringe instilled into the stomach and allowed to remain in contact with the stomach for 1 min, then it was aspirated and the area rinsed with sterile saline. The area exposed to acetic acid was 60 mm2. The rats were killed by cervical dislocation and the stomach was removed and pinned out on a wax block. A paper grid with an area of 25 mm2 was placed alongside the ulcer, which was then photographed (Figure 3). We then determined the ulcer area by planimetry, using × 5 enlargements of the photographs.
The area of ulceration in pixels was converted to square millimeters using the paper grid as a reference. Previous work using this ulcer model has revealed that ulcers induced by acetic acid are characterized by a thick layer of granulation tissue at the base and glandular disorganization at the ulcer margins. The ulcers involved the full thickness of the mucosa and penetrated into the muscularis mucosa. Perforation was not observed with this model. The rats were infected by giving the H pylori inoculum orally every alternate day for seven days.
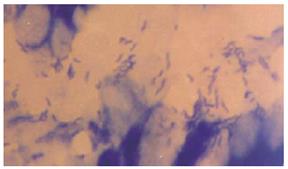
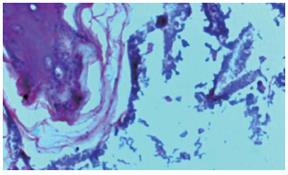
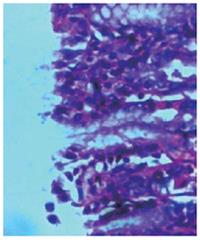
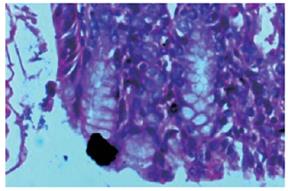
Bacterial content of ulcers: To determine the bacterial content in gastric ulcers, animals were killed under aseptic conditions. Tissue (150 mg) was washed in sterile PBS and transferred to sterile, pre-weighed containers (to determine sample weight), sterile PBS was added, and then the sample was homogenized. Serial dilutions were then plated on Mc-Conkey agar and tryptic soy agar plates and incubated for 18-24 h under aerobic conditions. The colony-forming units (CFU) were determined using a Leica colony counter. Results are expressed as CFU per gram of tissue. Tissue samples of the ulcers and healthy tissues were embedded in plastic using a commercially available kit (JB-4 embedding kit; Polysciences, Warrington, PA). Thin sections (1-1.5 μm) were stained with methylene blue-fuchsin (basic) and examined under a light microscope (Figures 4 and 5).
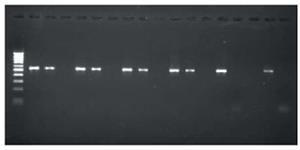
Further, the stomach tissue was collected for determination of ulcer size, histopathology and molecular confirmation of H pylori using Polymerase Chain Reaction (PCR) (Figure 6).
The rats were then divided into two treatment groups, GroupIof 6 rats who orally received methanolic extract of S. mukorossi (Figure 7) and Group II of 6 rats who orally received ethanolic extract of R. emodi (Figure 8). Group III of 6 rats received saline or dextrose. The total daily intake of dried Smukorossi and Remodi was 2.5 mg/d and 3.0 mg/d respectively for seven days.
The effect of treatment with the extracts of S. mukorossi and R. emodi was assessed. Ulcers were induced in the rats as described above. Seven days after ulcer induction and H pylori infection, both groups of rats were orally given the prescribed dose of both the extracts of 2.5 mg/mL and 3.0 mg/mL for seven days. The effect of treatment was checked by sacrificing the rats by cervical dislocation, removing the stomach and photographing it for ulcer determination, and taking tissue samples for bacterial culturing.
The data were analyzed with a chi-square (χ2) test.
In the in vitro study, we found that S. mukorossi and R. emodi extracts exert an inhibitory effect on H pylori (Figure 9). Ethanol and chloroform extracts of S. mukorossi and benzene and ethanol extracts of R. emodi with various concentrations ranging from 10-200 μg/mL were tested for their effect on H pylori growth by the disk diffusion method. In the in vivo study, we found that the extracts of S. mukorossi and R. emodi were able to clear H pylori infections in the rat models at concentrations of 2.5 mg/mL and 3.0 mg/mL post-infection (Figures 7 and 8).
It was found that, in the 30 isolates tested, the strains showed approximately 20 mm and 19 mm zones of inhibition with very low (10 μg/mL) concentrations of the ethanol extract of S. mukorossi and the benzene extract of R. emodi. The chloroform extract of S. mukorossi and the ethanol extract of R. emodi showed approximately 18 mm and 15 mm zones of inhibition at 10 μg/mL concentration. Maximum inhibition zones of approximately 98 mm and 94 mm were attained with the extracts of S. mukorossi, and maximum inhibitions zones of 92 mm and 90 mm were attained with the extracts of R. emodi at a concentration of 200 μg/mL. The details of the MIC of both the extracts against H pylori are listed in (Figure 9). In vivo, we found that the extracts of S. mukorossi and R. emodi were able to clear H pylori infections at the concentrations of 2.5 mg/mL and 3.0 mg/mL post-infection (Figures 7 and 8).
Elimination of H pylori has been a major objective of treatment strategies worldwide. Unfortunately, none of them have been able to achieve 100% eradication rates[24]. The main reason underlying this failure could be the rapidly emerging resistance among many strains of H pylori towards various antibiotics. In addition to increased acquired resistance with the majority of antibiotic therapies, which are considered highly efficient for long-term treatment, studies have shown non-compliance among patients.
Apparently related to the incomplete cures achieved with the triple therapies and their possible side effects, herbal systems of medicine have become increasingly popular in recent years. According to one estimate, nearly 70% of synthetic drugs are derived from medicinal herbs and medicinal herbs have figured high in pharmaceutical industry research because of their high therapeutic activity. Herbal therapies have shown better efficiency and efficacy in inhibiting H pylori at both in vitro and in vivo levels[25,26]. Based on the above, we decided to look for plant extracts capable of inhibiting the growth of H pylori. The reason for selecting S. mukorossi and R. emodi to assess antibiotic activity against H pylori is their chemical nature. The chemistry of these herbs is very complex and involves large amounts of physiologically active compounds. Not all the constituents present in the plant have therapeutic activity. Physiological activity is largely dependent on the complete structure, including the glycosidic moiety[27]. This activity is enhanced several-fold due to the presence of sugars in these compounds[28]. S. mukorossi and R. emodi are saponins and anthraquinones in nature and it is because of this that these plants exhibit antibacterial activity.
Among various extracts of both these plants, we found chloroform and ethanolic extracts of S. mukorossi exerted strong antibacterial effects on all the gram +ve and gram -ve organisms, including H pylori, while the petroleum ether and benzene extracts did not show any effect. Similarly, benzene and ethanolic extracts of R. emodi were found to exhibit a strong inhibitory effect on H pylori and other organisms while the petroleum ether and chloroform extracts showed no potential affect either on H pylori or other gram +ve and -ve microbes. Our study is the first to report the antibacterial effect of these plant extracts on H pylori. In our screening, we found that all 30 H pylori isolates were sensitive to the test compounds. As evident from the results, the compounds tested were found to possess antibacterial activities on H pylori and were not affected by the resistance of the organism to antibiotics.
The sensitivity of H pylori to commonly used antibiotics by (E-test) was carried out to assess susceptibility patterns and the sensitivity of the compounds was studied to evaluate the anti-H pylori activity of our extracted compounds (10-200 μg/mL by disk diffusion method). We assessed the minimum inhibitory dose of the compounds at which the survival of H pylori was in jeopardy. As evident from Figure 9, the extracts of S. mukorossi and R. emodi are highly effective even at a very minimal dose of 10 μg/disk.
Further, we found that none of the isolates acquired resistance even after 10 passages at sub-inhibitory concentrations. We found that S. mukorossi extracts and R. emodi extracts of 10 μg/mL concentration proved to be the MIC for almost all the isolates (except MS-24 and MS-28, which were found to be completely inhibited at 8 µg/mL). The reason behind this could be strain variation (data not shown).
S mukorossi and R. emodi extracts were found to inhibit all strains of H pylori (both sensitive and resistant) at a concentration of 10 μg/mL. This inhibitory concentration is comparable with the previously reported allicins 6-12 μg/mL, garlic oil 8-32 μg/mL[29] and ajoenes 10-25 μg/mL of oil macerated garlic extract. Our compounds proved to be more potent than thiosulfinate 40 μg/mL, vinyldithiins < 100 μg/mL, epigallocatechin gallate in Chinese lung chen tea 50-100 μg/mL and garlic powder 250 μg/mL[29]. Further more, the organism did not develop any resistance to the test compound even after 10 subsequent passages, grown at sub-inhibitory concentrations, whereas H pylori strains acquired resistance to amoxycillin and clarithromycin after 10 sequential passages[30,31]. In addition, we found that our extract had almost equivalent bacterial activities against both antibiotic susceptible and resistant H pylori strains. Animal studies suggest that an extract of S. mukorossi and R. emodi taken orally may help prevent stomach ulcers and it is of interest to note that S. mukorossi and R. emodi appear to be both anti-inflammatory and anti-ulcerogenic. However, future studies concerning these properties must be carried out to justify their anti-ulcerogenic properties.
In conclusion, the results presented here indicate that the extracts of S. mukorossi and R. emodi, which were screened for their anti-bacterial activity against H pylori, are active both in vitro and in vivo. In addition, the in vivo studies also proved to be highly efficient in terms of dosage, tolerability and curing active H pylori infection. Future studies will assess the mechanism by which these extracts effect the survival of H pylori.
We are very grateful to Dr. CM Habibullah for his patronage, encouragement, guidance and financial support during the course of the study.
S- Editor Liu Y L- Editor Lutze M E- Editor Liu WF
| 1. | Kuipers EJ. Review article: exploring the link between Helicobacter pylori and gastric cancer. Aliment Pharmacol Ther. 1999;13 Suppl 1:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 171] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 2. | Bazzoli F, Zagari RM, Fossi S. Short-term low dose triple therapy for the eradication of Helicobacter pylori. Eu J Gastroenterol Hepatol. 1994;6:773-777. [DOI] [Full Text] |
| 3. | Lamouliatte H, Cayla R, Talbi P, Zerbib F, Megraud F. Randomised study comparing two seven days triple therapies with lansoprazole and low dose of clarithromycin plus Amoxycillin or tinidazole for Helicobacter pylori eradication. Gastroenterol. 1996;110:A170. |
| 4. | Lind T, Veldhuyzen van Zanten S, Unge P, Spiller R, Bayerdörffer E, O'Morain C, Bardhan KD, Bradette M, Chiba N, Wrangstadh M. Eradication of Helicobacter pylori using one-week triple therapies combining omeprazole with two antimicrobials: the MACH I Study. Helicobacter. 1996;1:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 394] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 5. | Megraud F, Lehn N, Lind T. The MACH 2 Study. Helicobacter pylori resistance to antimicrobial agents and its influence on clinical outcome. Gastroenterol. 1997;112:A216. |
| 6. | Thyagarajan SP, Ray P, Das BK, Ayyagari A, Khan AA, Dharmalingam S, Rao UA, Rajasambandam P, Ramathilagam B, Bhasin D. Geographical difference in antimicrobial resistance pattern of Helicobacter pylori clinical isolates from Indian patients: Multicentric study. J Gastroenterol Hepatol. 2003;18:1373-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Results of a multicentre European survey in 1991 of metronidazole resistance in Helicobacter pylori. European Study Group on Antibiotic Susceptibility of Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 1992;11:777-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 94] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Pandey G. Dravyaguna Vijanana; v I. Varanasi. India: Krishnadas Academy 1998; 191-196. |
| 9. | Kirtikar KR, Basu BD. Indian Medicinal Plants; v I. Allahabad. India: B. L. M. Basu Publ 1991; 633. |
| 10. | The Useful Plants of India. Publication and Information Directorate. New Delhi: IR 1986; 547. |
| 11. | Rastogi RP, Mehrotra BN. Compendium of Indian Medicinal Plants; v 2. New Delhi: CDRI Publication 1999; 609-610. |
| 12. | Dwivedi AK, Chaudhary M & Sarine JPS. Standardisation of a new spermicidal agent sapindus saponin and its estimation in its formulation. Indian J Pharm Sci. 1990;52:1657. |
| 13. | Castleman M. The Healing Herbs: The Ultimate guide to the curative powers of nature's medicine. Emmaus, PA: Rodale Press 1991; 305-307. |
| 14. | Peirce A. The American Pharmaceutical Association practical guide to natural medicines. New York: William Morrow and Company Inc 1999; . |
| 15. | Borgia M, Sepe N, Borgia R, Ori-Bellometti M. Pharmaco-logical activity of an herbal extract: controlled clinical study. Curr Ther Res. 1981;29:525-536. |
| 16. | Duke JA. Green Pharmacy. Emmaus, PA: Rodale Books 1997; 507. |
| 17. | Wang X, Lous Z, Mikage M, Namba T. Pharmacognostical studies on the Chinese crude drug da-huang rhubarb II Botanical origin of three unofficial da-huang. Shoyakugaku Zasshi. 1988;42:302-309. |
| 18. | Wang X; AOAC. K Helrich, editor. In: Official Methods of the Association of Official Analytical Chemists. 15th ed 1990; 762. |
| 19. | Wang X; NCCLS. Methods for dilution Antimicrobial susceptibility tests for bacteria that grow aerobically. 3rd ed. Approved standard. In: Official Methods of the Association of Official Analytical Chemists. 15th ed 1993; M7-A3. |
| 20. | Wang X; NCCLS. Methods for dilution Antimicrobial susceptibility tests of anaerobic bacteria. 3rd ed. Approved standard. In: Official Methods of the Association of Official Analytical Chemists. 15th ed 1993; M11-A3. |
| 21. | Zaika LL. Spices and herbs: their antimicrobial activity and its determination. J Food Safety. 1988;9:97-118. [RCA] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 224] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 22. | Ali SM, Khan AA, Ahmed I, Musaddiq M, Ahmed KS, Polasa H, Rao LV, Habibullah CM, Sechi LA, Ahmed N. Antimicrobial activities of Eugenol and Cinnamaldehyde against the human gastric pathogen Helicobacter pylori. BMC-Annals of Clinmicrob & Antimicrobial. In: Official Methods of the Association of Official Analytical Chemists. 15th ed 2005; Available from: http:// www.ann-clinmicrob.com/content/4/1/20. |
| 23. | Okabe S, Pfeiffer CJ. Chronicity of acetic acid ulcer in the rat stomach. Am J Dig Dis. 1972;17:619-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Moshkowitz M, Konikoff FM, Peled Y, Santo M, Hallak A, Bujanover Y, Tiomny E, Gilat T. High Helicobacter pylori numbers are associated with low eradication rate after triple therapy. Gut. 1995;36:845-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | O'Gara EA, Hill DJ, Maslin DJ. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl Environ Microbiol. 2000;66:2269-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 196] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Tabak M, Armon R, Neeman I. Cinnamon extracts' inhibitory effect on Helicobacter pylori. J Ethnopharmacol. 1999;67:269-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 78] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Vladimir K, Ludmila M. Glycosides in Medicine: "The Role of Glycosidic Residue in Biological Activity. Curr Med Chem. 2001;8:1313-1318. |
| 28. | Albrecht HP. In: naturally occurring glycosides; Ikan R, editor. Chichester, New York: John Wiley & sons, Ltd 1999; 83-123. |
| 29. | Goodwin CS. Antimicrobial treatment of Helicobacter pylori infection. Clin Infect Dis. 1997;25:1023-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Sörberg M, Hanberger H, Nilsson M, Björkman A, Nilsson LE. Risk of development of in vitro resistance to amoxicillin, clarithromycin, and metronidazole in Helicobacter pylori. Antimicrob Agents Chemother. 1998;42:1222-1228. [PubMed] |
| 31. | Dore MP, Osato MS, Realdi G, Mura I, Graham DY, Sepulveda AR. Amoxycillin tolerance in Helicobacter pylori. J Antimicrob Chemother. 1999;43:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 2.7] [Reference Citation Analysis (0)] |