INTRODUCTION
Dendritic cells (DCs) play a pivotal role in the initiation of immune responses and are considered as important targets for effective immunotherapeutic strategies against cancer and infectious diseases[1,2]. DCs acquire antigen in peripheral tissues, migrate to peripheral lymphoid tissue and subsequently present MHC-peptide complexes to naive T cells for the induction of immune responses. In this scheme, DCs are also believed to control the type of immune response that is generated through the secretion of cytokines such IFN-γ or IL-4 which regulate Th1 or Th2 responses, respectively. The development of DC and their activation state is dependent upon growth factors, such as fms-like tyrosine kinase 3 ligand (Flt-3L) and granulocyte macrophage colony-stimulating factor (GM-CSF). When administered in vivo in the form of recombinant proteins, these cytokines can dramatically alter the absolute number of DC and the subset composition of lymphoid tissue and blood-borne DC[3,4]. This has important implications for the use of these cytokines as adjuvants, since recent evidence suggests that distinct DC subsets are responsible for the generation of different classes of immune responses. Monocyte-derived CD11c+ DCs induce T cells to produce Th1 cytokines in vitro, whereas the CD11c- plasmacytoid T cell-derived DCs elicit the production of Th2 cytokines. Flt-3L can increase the number of cells in both subsets of DC, but GM-CSF only increases the CD11c- precursors[3,4]. A number of studies have shown that recombinant Flt-3L protein can augment in vivo immune responses and that this cytokine has immunotherapeutic potential[5-7]. It is important to establish if the adjuvant effects of Flt-3L can be achieved by plasmid DNA immunization, which would provide a more versatile delivery system for antigen delivery in vaccination protocols that involve this cytokine[8].
Direct intramuscular or intradermal injection of plasmid DNA generates potent cell-mediated and humoral immune responses against a variety of pathogens, such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), ebola virus, influenza virus, mycobacteria and malaria in animal models of these diseases[9-11]. Genetic vaccines induce antigenic expression that resembles a response to native viral epitopes more closely than do other conventional vaccines. Live attenuated or killed vaccines are often altered in protein structure and antigenicity and may be rendered less efficient at stimulating protective immunity. An important feature of DNA-based immunization is the induction of cytotoxic T cells (CTL) that recognize and lyse virus-infected cells, thereby limiting viral spread. Recent DNA-based immunization studies have reported the induction of anti-HIV CTL activity and anti-plasmodium T cell responses in chronically infected individuals who were immunological naïve for these pathogens[12,13]. Several lines of evidence implicate DC as the principal antigen-presenting cell (APC) in mediating effective immune responses that involve plasmid DNA vaccination. Firstly, bone marrow-chimeric mice have shown that bone marrow-derived APCs are responsible for the induction of the immune response after DNA vaccination[14,15]. Secondly, DCs but not B cells or keratinocytes, isolated from DNA-vaccinated mice were able to present the immunizing antigen to T cells in vitro[17]. Thirdly, injection of DNA leads to the direct transfection of DCs in vivo[1]. Finally, direct in vivo visualization of antigen-expressing DCs from draining lymph nodes after gene-gun vaccination has been demonstrated[18]. Collectively, these reports indicate plasmid DNA immunization with a DC-specific adjuvant is likely to lead to a potent immune response.
Hepatitis C virus (HCV) infection is the major causative agent of transfusion-associated and community-acquired non-A or non-B hepatitis. An estimated 170 million individuals are infected with HCV worldwide and this represents a viral pandemic[19]. Infection with HCV is associated with high morbidity and mortality, since 75%-85% of individuals infected with HCV develop chronic infection and hepatitis whilst approximately one third progress to cirrhosis and eventual liver failure[20]. Moreover, HCV-infected individuals are at increased risk for hepatocellular carcinoma (HCC) within 10-20 years of infection[21]. An effective therapeutic and/or prophylactic anti-HCV vaccine is, therefore, of considerable clinical importance[22]. We have previously identified various HCV structural and non-structural proteins that may be used for vaccination studies designed to limit HCV pathogenesis[23-25]. Here we have shown that co-immunization of DNA encoding GM-CSF and Flt-3L, together with DNA encoding HCV viral antigen leads to enhanced humoral and cellular immune responses that collectively provide protective immunity against HCV-induced pathogenesis in mice.
MATERIALS AND METHODS
Construction of DNA expression vectors
A plasmid coding for HCV NS5 was cloned as described in detail previously[25]. pcDNA3-Flt3-L was cloned from a plasmid PCRblunt-mFlt3-L encoding the leader sequence and the extracellular domain of mouse Flt3-L. PCR amplification was performed with the following primer pair: in sense direction 5´-CGGGGTACCATGACAGTGCTGGCGCCAGCCTGG-3´ (Kpn I site and engineered start codon ) and in antisense direction 5´-GCTCTAGAAAGCTTTTATTAATGGTGATGATGGTGATGCTGCCTGGGCCGAGGCTC-3´ (XbaIand Hind III site, engineered stop codons and 6xHis Tag) with a proof reading high fidelity system (Roche, Germany) and a standard PCR protocol as follows: Pre-denaturation at 95°C for 2 min; 30 amplification cycles, each cycle consisting of denaturation at 95°C for 1 min, primers annealing at 57°C for i min and extension at 72°C for 1 min; and finally an extra incubation at 72°C for 7 min to ensure full extension of the products. After restriction enzyme digest with KpnIand XbaIfor 20 h, the insert was cloned into a standard mammalian transfection vector with a CMV promoter and a given number of CpG motifs (pcDNA3; Invitrogen, Groningen, Netherlands)). Standard sequencing was performed to verify correct nucleotide sequence (Sequence Laboratories, Göttingen, Germany). Cloning and characterization of pGM-CSF was described elsewhere[25]. Plasmids were grown in DH5α cells, and plasmid DNA was subsequently purified by Qiagen Giga Kit using the endofree buffer system (Hilden, Germany).
HCV infection model
In the absence of a small animal model for HCV infection, we used a tumor model expressing a target HCV antigen (HCV-NS5). A stable HCV NS5-expressing mouse myeloma cell line SP2-NS5-21 (H-2d background) syngenic to BALB/c mice was established by electroporation and limiting dilution, followed by Western blot analysis for HCV NS5 expression. BALB/c mice inoculated with 2 × 106 syngenic myeloma cells (SP2-NS5-21) into the flank develop visible tumors within 7 d and succumb to terminal tumor burden within 15-25 d. The tumors constitutively express HCV NS5 protein and are an established model of HCV viral challenge that has been characterized previously[25].
In vitro expression
A human hepatoma cell line (HuH-7) was transiently transfected with the various plasmids pcDNA-Flt3-L, pcDNA3-NS5 and pcGM-CSF to verify protein and antigen expression as determined by Western blot and ELISA. Detail protocols for immunoblot for NS5 and for Western blot and ELISA for GM-CSF are described previously[23,25]. Flt3-L expression was confirmed by Western blot technique with an anti-His-Tag antibody and a mouse Flt3-L-specific ELISA (R&D, Minneapolis, MN). Cell supernatants were collected 48 h after standard calcium phosphate transfection and cell lysates were prepared in a RIPA buffer (50 mmol/L Tris, 150 mmol/L NaCl, 10 mL/L Nonidet P-40, 5 g/L sodium deoxycholate, 10 g/L SDS). Proteins were lysed and supernatants were loaded onto a 150 g/L-SDS-PAGE gel and blotted onto nitrocellulose membranes. After blocking with 30 g/L BSA in TBS for 2 h, membranes were incubated for 1 h with an anti-His-Tag antibody (Quiagen, Hilden, Germany) in TBS at room temperature (dilution 1:1000), followed by an incubation with sheep-anti-mouse Ig horseradish peroxidase antibody (Amersham Pharmacia, Freiburg, Germany) at 1:1000 dilution. Chemiluminescence detection was performed with the ECL system following the manufacturer’s protocol (Amersham Pharmacia). Flt3-L ELISA was performed with a commercially available ELISA kit (R&D, Minneapolis, MN). Lysates and supernatants were used undiluted. To analyze Flt3-L serum levels of immunized mice, a 1:50 dilution of serum collected through tail vein bleeding was used as first antibody in this assay.
Plasmid DNA Immunization
BALB/c (H-2d) and C57/BL6 (H-2b) mice were purchased from Charles River Laboratories and maintained under standard pathogen-free conditions in the animal facility (Zentrales Tierlabor) at our institution (University of Heidelberg). Five days prior to plasmid immunization, 100 μL of 50 μg/mL Flt3-L or mock plasmid DNA was injected into the quadriceps muscle over five different sites. Plasmid immunization was performed three times thereafter (100 μg plasmid DNA in 100 μL 9 g/L NaCl) at bi-weekly intervals (administration into the opposite leg). Empty plasmid vector (mock) immunization was employed as a negative control. Animals (n = 5) were injected with pcDNA3-NS5 (50 μg) alone or in combination with an Flt3-L (50 μg) or GM-CSF (50 μg) plasmid. Furthermore, one group was immunized with 50 μg of HCV NS5 and Flt3-L combined with GM-CSF (both 25 μg) to study the combined effect of both cytokines. One group was immunized subcutaneously into the footpad. Mice were sacrificed 14 d after the last immunization event with collection of spleen cells and serum to determine humoral and cellular immune responses. The immunization experiments were once repeated; each immunization group contained at least 5 animals.
Measurement of humoral immune responses
Anti-HCV NS5 antibody level in the serum of each immunized animal was determined by ELISA. In brief, microtiter plates (Nunc Maxisorp) were coated with 0.5 μg/well recombinant HCV NS5-4 protein (Mikrogen, Munich, Germany), incubated overnight at 4°C and blocked with foetal bovine serum (FBS) for 2 h at 20°C. A 1:50 dilution of mouse serum was added to the plates, incubated for an additional 1 h at 20°C, and washed 4 times with phosphate buffered saline (PBS) containing 5 mL/L Tween-20. A peroxidase-conjugated AffinePure goat anti-mouse IgG (Dianova, Hamburg, Germany) was applied to the plates at a 1:2000 dilution, incubated for 1 h, washed, and substrate (OPD) was added for color development (Abbott).
Lymphoproliferation and cytokine release assays
Mice were anesthetized with diethylether for harvesting of spleen cells. Erythrocytes were removed by incubation in 8.3 g/L NH4Cl / 0.17 mol/L Tris (pH 7.4) for 5 min at 25°C. Spleen cells were washed 2 times and cultured in triplicate using 96-well round bottom plates at 5 × 105 cells/well in 200 μL DMEM (Cellgro Mediatech, Washington, DC) containing 100 mL/L FBS and 2-mercaptoethanol (5 × 10-5 mol/L). Cells were stimulated for 3 d with recombinant HCV NS5-4 protein (aa 2622–2868) (Mikrogen, Munich, Germany) at various concentrations (0, 0.01, 0.1 and 1 μg/mL). Following the addition of radioactive 3H-thymidine (1 μCi/well), cells were incubated for an additional 18 h, and 3H-thymidine uptake into DNA was measured after harvesting; incorporation of radioactivity was corrected for background activity (∆ cpm). To determine cytokine secretion levels of lymphoproliferative cells stimulated with recombinant HCV NS5-4 protein, cells were cultured as described, and mIFN-γ and mIL-4 levels were measured by commercial kits according to manufacturer’s instructions (Pharmingen, San Diego, CA).
ELISPOT assay
To assess the number of IFN-γ-secreting cells at the individual cell level, single cell suspensions from spleens harvested from immunized mice mouse were analyzed in an IFN-γ ELISPOT assay. Cells were directly assessed in this assay without prior in vitro expansion in the presence of 1 μg/mL recombinant NS5-4 protein at 37°C (50 mL/L CO2) in IFN-γ-bound microtiter plates to measure IFN-γ cytokine secretion as means of CD8+-T-cell function (AID, Straßberg, Germany). After washing with PBS-Tween buffer, cells were incubated with a secondary antibody suspended in DMEM supplemented with 100 mL/l FCS and 2-mercaptoethanol.
Assessment of CTL activity in vivo
BALB/c mice were immunized im three times with either mock DNA or NS5 vector and co-immunized with Flt3-L or Flt3-L and GM-CSF. One week after the last immunization event with 2 × 106 syngenic SP2/0-derived cells stably expressing NS5 were washed (Sp2-NS5-21), resuspended in 200 μL of PBS, and inoculated sc into the right flank. SP2/0 cells that stably expressed HCV core protein (SP2/19) were used as a control in selected animals. Tumor formation, tumor size, mouse weight and mouse survival was assessed at distinct intervals post inoculation.
RESULTS
In vitro expression of Flt-3L from Flt-3L-pcDNA3
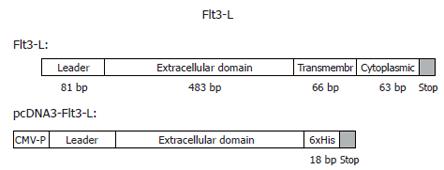
Flt-3L is type I transmembrane, non-disulphide linked, homodimer glycoprotein of approximately 30 ku. This cytokine may also be found as a soluble form in serum that comprises only the extracellular domain of approximately 160 amino acids that retains full biological activity[26,27]. We, using PCR, have cloned Flt-3L DNA encoding the leader sequence and extracellular domain, together with a hexahistidine C-terminal tag, into the eukaryotic expression vector pcDNA-3 for use as a Flt-3L delivery system. Figure 1 shows a schematic representation of the major domains of full-length and truncated forms of Flt-3L. DNA encoding the leader and extracellular domains of Flt-3L was generated by PCR and ligated into the eukaryotic expression vector pcDNA3 to generate Flt-3L-pcDNA3. Restriction digest and DNA sequence analysis confirmed the presence of the predicted insert size and its correct orientation (data not shown).
Figure 1 Cloning of the soluble form of mouse Flt3-L containing the leader and the extracellular domain into a mammalian expression vector with CMV-promoter and RSV-enhancer.
The C-terminus was tagged with a 6xHis. The soluble form of mFlt3-L is fully active.
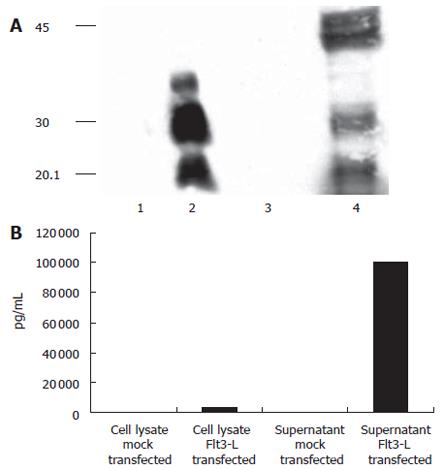
Figure 2 shows that Flt-3L-pcDNA3 was authentically expressed after transient transfection into HuH-7 cells. Figure 2A shows a Western blot of Flt-3L-pcDNA3-transfected HuH-7 cell lysate and culture supernatant probed with an anti-His monoclonal antibody. Anti-His reactive protein bands of 20 – 30 ku were present in both cell lysate and supernatant of cells transfected with Flt-3L-pcDNA3. This protein band pattern most likely represents the different glycosylation forms of Flt-3L which have been described previously[27]. Additional bands of 40 – 42 ku were detected in the cell culture supernatants that may represent the dimeric form of Flt-3L. No reactivity with the anti-His monoclonal antibody was seen in either cell lysate or culture supernatants from HuH-7 cells transfected with control vector. Further confirmation that Flt-3L was expressed from Flt-3L-pcDNA3 was provided by ELISA using an anti-Flt-3L specific monoclonal antibody with samples from Flt-3L-pcDNA3-transfected HuH-7 cells as substrate. Figure 2B shows that Flt-3L could be detected in cell lysate and culture supernatant from Flt-3L-pcDNA3-transfected cells, whereas no reactivity was detected in equivalent samples from control transfected cells. This data demonstrates that Flt-3L protein could be generated from Flt-3L-pcDNA3.
Figure 2 Expression of Flt3-L in transiently transfected HuH-7 cells: (A) Detection of mouse Flt3-L on a 150 g/L SDS-PAGE using an anti-His-Tag antibody.
Different glycosylation forms of Flt3-L in cell were detected in cell lysates and supernatants. Lane 1 and 3: mock-transfected cells; lane 2 and 4: Flt3-L-transfected cells; lane 1 and 2: supernatants after transient transfection; lane 3 and 4: cell lysates. (B) To confirm these results, supernatants and cell lysates were incubated by an Flt3-L-specific ELISA. Again, secretion levels into the supernatant were more than 20-fold higher compared to levels seen in the cell compartment.
Flt-3L- or GM-CSF-pcDNA3 enhances anti-HCV immune responses
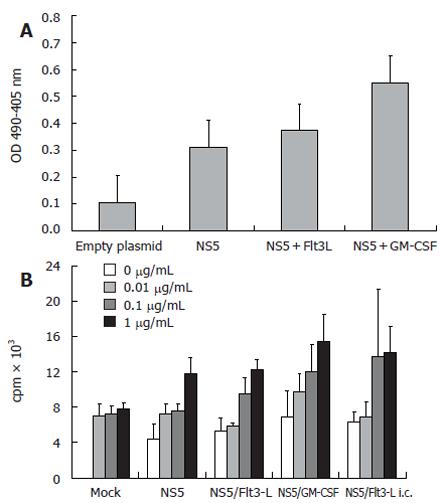
Immune responses in mice to the HVC structural protein NS5 in the presence or absence cytokines that regulate dendritic cells were examined. Mice were immunized intramuscularly three times, at bi-weekly intervals, with pcDNA3-NS5 in combination with either Flt-3L-pcDNA3 or GM-CSF-pcDNA3. Serum samples from vaccinated mice were investigated for the presence of anti-HCV antibodies by ELISA using as substrate, the peptide NS5-4 which comprises amino acids 2622-2868 of the protein NS5. The data in Figure 3 shows that co-immunization of pcDNA3-NS5 with Flt-3L-pcDNA3 led to elevated levels of anti-HCV antibody compared to that found when pcDNA3-NS5 was administered alone. The level of anti-HCV antibody was further increased when pcDNA3-NS5 was co-immunized with GM-CSF-pcDNA3. Similar results were obtained using either BALB/c or C57/BL6 mice (data not shown). These experiments showed that administration of plasmid DNA encoding cytokines that regulate the development and maturation of dendritic cells, at the same time as inoculation with DNA encoding HCV protein, can produce elevated anti-HCV humoral immune responses. The adjuvant effect of Flt-3L or GM-CSF did not appear to affect the level of anti-HCV-specific T cell proliferation not readily evident at the level of T cell activation and proliferation. Spleen T cells from NS5 plasmid DNA-immunized mice were stimulated in vitro with recombinant HCV NS5-4 peptide and the subsequent proliferative response measured by 3-H-thymidine uptake. The data in Figure 3 shows that there was no significant difference in anti-NS5 HCV T lymphocyte proliferation between cells isolated from mice immunized with NS5-pcDNA3 alone or in combination with either Flt-3L pcDN3 or GM-CSF-pcDNA3. In addition, when IFN-γ and IL-4 levels in the supernatant of stimulated T cells were measured by ELISA, there were no significant differences between any of the immunization groups (data not shown).
Figure 3 A: Anti-HCV NS5 ELISA showing mean levels of specific antibodies after genetic immunization with HCV-NS5-expressing plasmid.
Controls included wells coated with BSA and sera derived from mock-immunized mice. Each group comprised 10 BALB/c mice and mice sera were pooled before the assay. ELISA plates were coated with HCV-NS5-4, therefore, antibody levels may be underestimated; B: T-cell proliferation assay (n = 10 mice/group) with different amounts of stimulating recombinant HCV NS5-4 protein (0.01-1 µg/mL). An increase of thymidine incorporation was seen after stimulation with 0.1 and 1 µg/mL recombinant protein. However, levels of T-cell proliferation after co-immunization with Flt3-L were only slightly higher than in NS5-immunized mice. Note that stimulation with a non-relevant protein (HBsAg) induced only background activity, demonstrating the antigen specificity (data not shown).
Protective immunity generated by Flt-3L and GM-CSF
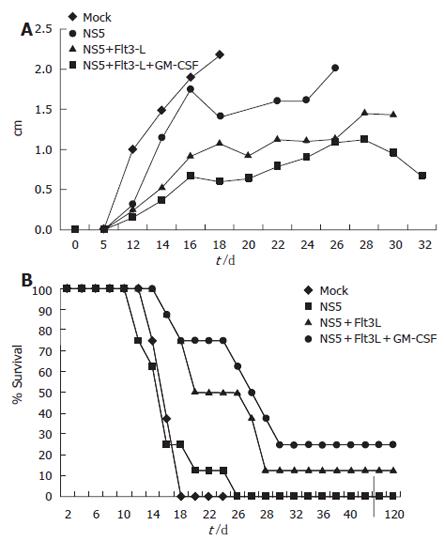
In vivo CTL activity was assessed by a tumor model. Ten days after the last immunization event, animals were challenged in the right flank with a HCV-NS5 mouse myeloma cell line. Eighteen days after challenge, all mock plasmid-immunized animals were dead. Animals immunized with 50 μg of HCV-NS5 plasmid lived slightly longer, but all animals died by d 26. Co-vaccination with Flt3-L or Flt3-L and GM-CSF lead to survival and complete protection in some animals: 25% and 10%, respectively. Tumor size was also significantly reduced in co-immunized animals (Flt3-L and combination of Flt3-L and GM-CSF). Mice immunized with the same syngenic SP2/0 cell line expressing a different HCV structural protein (HCV core) as a control died as well by d 17, like mock-immunized animals. Indeed, 100% of mice immunized with mock DNA or challenged with SP2/19 cells demonstrated tumor formation, confirming the specificity of the cytotoxic activity in this tumor model (Figure 4).
Figure 4 Tumor model to assess CTL activity generated in vivo after genetic vaccination.
Mice were immunized three times with a total of 100 μg of plasmid DNA. Each vaccination group (n = 8) was challenged with 2 × 106 syngenic mouse myeloma cells stably expressing HCV-NS5 protein in the right flank. Mice developed notable tumors around d 8-10. (A) Tumor size in different immunization groups. Note that mice had to be sacrificed at maximum tumor diameter size of 2 cm. Sacrificed mice were then taken out from the measurement. (B) Mouse survival after genetic vaccination in each group. Three mice were long-term survivor and never developed significant tumor burden.
In vitro and in vivo CTL activity: ELISPOT and tumor model
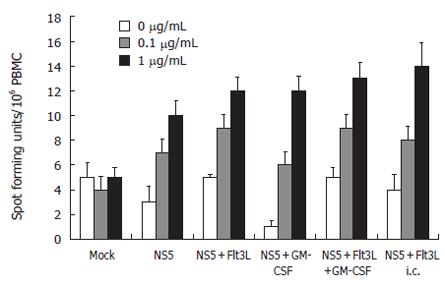
We used IFN-γ-bound microtiter plates to measure IFN-γ cytokine secretion as means of CD8+-T cell function in an ELISPOT on an individual cell level. Spleen cells were harvested and cultured overnight in the presence or absence of 1 mg/L recombinant NS5-4 protein. Flt3-L, GM-CSF, the combination of both cytokines injected each with smaller amounts of DNA (25 μg per vaccination) and the subcutaneous injection with Flt3-L resulted in comparable levels of NS5-4-specific IFN-γ-producing T cells. The number of cells was significantly higher compared to NS5-immunized animals alone and the mock-injected animals (Figure 5).
Figure 5 ELISPOT assay: Assessment at the individual single cell level of the number of mIFN-γ-secreting cells as means of CD8 T cell function.
Spleen cells were stimulated with recombinant HCV-NS5 protein at 0.1 and 1 µg/mL, and the number of spot forming units/106 PBMC was counted after 24 h incubation period (n = 10 mice/group). Note that lymphocytes were not restimulated in NS5-expressing cells.
In vivo expression of Flt3-L from Flt3-L- pcDNA3
In studies by He et al where Flt-3L DNA has been administered by hypodynamic needle, levels of serum Flt-3L have been raised 10-fold compared to control values. To determine whether Flt3-L-pcDNA3 administration in vivo also led to elevated levels of serum Flt-3L, mice were inoculated with this vector by intramuscular injection and serum samples harvested at 3, 6, 12, 24 h and at 3 d and 7 d post-inoculation. Serum samples were assessed for Flt-3 L by Flt-3L-specific ELISA. On no occasion, using either BALB/c or C57/BL6 mice, were the levels of serum Flt-3L significantly increased compared to control mice (data not shown). In addition, there was no increase in the size of lymphoid tissue, such as the spleen (data not shown), as has been reported following administration of recombinant Flt-3L protein or Flt-3L DNA by hypodynamic needle.
DISCUSSION
DNA vaccination is an efficient and versatile mechanism for the delivery of antigen to the immune system. The mechanism of antigen delivery is believed to operate by direct transfection of dendritic cells with the inoculated DNA construct; plasmid expression within these cells and the subsequent processing and presentation of antigen to T cells[17]. Dendritic cells are potent antigen-presenting cells for naïve antigen-specific T cells and, therefore, have the capacity to initiate humoral and cellular immune responses, which is an essential requisite of any vaccination strategy[28,29]. A feature of plasmid DNA vaccination has been the reported longevity of expression of the DNA construct which may lead to evidence of immunity for periods greater than 12 mo[14]. This may represent continuous expression of the plasmid DNA construct within lymphoid tissue where antigen-presenting cells, such as dendritic cells and antigen-specific T cells, interact in the appropriate environment to allow the necessary cellular interactions that lead to an effective immune response[30]. Because of these features, plasmid DNA vaccination has been considered as an effective means to provide prophylactic or therapeutic immune responses against HCV infection[31]. However, several studies to date that have investigated plasmid DNA vaccination in animal models of human HCV infection have so far only demonstrated low antibody titers against the immunizing HCV proteins[23-25]. It is clear if plasmid DNA vaccination is to provide effective immunization against HCV infection that enhanced immune responses will need to be generated. In order to address this issue, we considered it appropriate to target dendritic cells in an attempt to promote enhanced plasmid DNA-induced anti-HCV immunity in mice.
In this study, we have successfully generated an enhanced immune response against a structural HCV protein by co-immunization with plasmid DNA-encoding cytokines that regulate development and maturation of dendritic cells. When HCV plasmid and Flt-3L DNA were co-injected, the resultant anti-viral antibody titer was significantly increased, and was further enhanced by co-immunization with GM-CSF plasmid DNA. These phenomena parallel what has been seen upon administration of recombinant Flt-3L and GM-CSF protein during immunization with protein antigens. Importantly, we have demonstrated that this enhanced plasmid DNA-based immunization is effective at providing protective immunity against HCV-based tumor formation. We used an in vivo tumor model that expressed a HCV non-structural protein. Control mice died after 18 d, whereas co-immunized mice survived to some extend, especially after co-administration of an Flt3-L and a GM-CSF plasmid underlining synergistic effects of both cytokines seen in in vitro studies[8]. This underlines the local and antigen-specific immune induction, because models of recombinant administration (up to 500 μg/kg per day) or virally transduction with Flt3-L DNA have also demonstrated inhibition of the growth of B16 melanoma, EL-4 lymphoma, murine leukemia, and C3L5 breast tumors in mice[32-35]. However, these models used large amounts of recombinant FLT3-L and repeated injections are required to induce these effects, unlike the relatively small amounts of plasmid DNA injection used in this study.
A feature of our study is that protective immunity against HCV-induced tumor formation was achieved in the apparent absence of any detectable increase in serum cytokine level from the inoculated plasmids. In humans, serum levels of Flt3-L are relatively low (< 100 pg/mL). In mice we measured serum levels around 300 pg/mL and did not find an increase after genetic vaccination with Flt3-L plasmid. These results are in contrast to data that have reported an initial increase in serum Flt3-L levels after a single injection of hydrodynamic human Flt3-L gene delivery when serum levels up to 40 μg/mL were observed and remained above 1 μg/mL for 6 d in mice[36]. In addition, elevated levels of serum Flt-3L, such as those following in vivo administration, resulted in an increase in the size of peripheral lymphoid tissues like spleen and lymph nodes. Furthermore, these elevated levels of Flt3-L were associated with significant increases in the number of DCs and NK-cells in various tissues, such as spleen and liver[36]. Our observations on the absence of any change in serum Flt3-L following plasmid DNA vaccination with this cytokine are in agreement with reports by others using other cytokines, such as IL-2, GM-CSF or IL-12, for plasmid DNA co-vaccination studies[23,37,38]. High levels of DC in a number of different organs may, under certain conditions, be associated with adverse side effects, including induction of haematopoietic malignant disease and the induction of autoimmunity[39]. Interestingly, a very recent study demonstrated the successful local recruitment of DC to the side of injection and the induction of CD4+ proliferative responses in a large animal model of a veterinary disease (calves) after co-vaccination with Flt3-L and GM-CSF plasmids, which has important implications of translating results from the small animal model mouse to men[40]. In this regard, a local increase in DC numbers with an accompanied enhancement of the immune response would be distinct clinical advantage and is demonstrated against HCV in our study. This approach of co-immunization with Flt3-L and GM-CSF has, therefore, important implications for the development of an effective antiviral HCV vaccine.