Published online Nov 21, 2006. doi: 10.3748/wjg.v12.i43.7042
Revised: July 25, 2006
Accepted: August 11, 2006
Published online: November 21, 2006
AIM: To construct a live attenuated Salmonella typhimurium (S. typhimurium) strain harboring the H pylori neutrophil activating protein (HP-NAP) gene as an oral recombinant DNA vaccine, and to evaluate its immunogenicity.
METHODS: By genetic engineering methods, the genomic DNA of H pylori was extracted as a template. The total length of the HP-NAP gene was amplified by polymerase chain reaction (PCR) and cloned into pBT vector for sequencing and BLAST analysis, then subcloned into a eukaryotic expression vector pIRES followed by PCR identification and restriction enzyme digestion. The identified recombinant plasmid pIRES-NAP was transfected into COS-7 cells for target fusion protein expression, and its antigenicity was detected by Western blotting. Then the recombinant plasmid was transformed into a live attenuated S. typhimurium strain SL7207 as an oral vaccine strain, and its immunogenicity was evaluated with animal experiments.
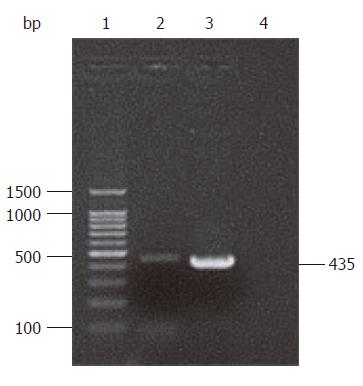
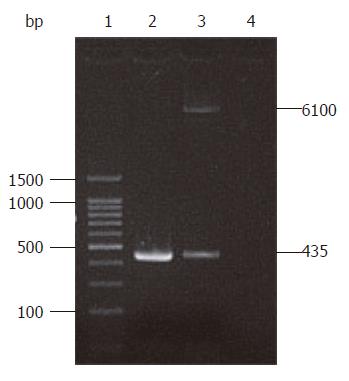
RESULTS: A 435 bp product was cloned using high homology with HP-NAP gene in GenBank (more than 98%). With identification by PCR and restriction enzyme digestion, a recombinant eukaryotic expression plasmid pIRES-NAP containing the HP-NAP gene of H pylori was successfully constructed. The expressed target protein had a specific reaction with H pylorii whole cell antibody and showed a single strip result detected by Western blotting. Oral immunization of mice with recombinant DNA vaccine strain SL7207 (pIRES-NAP) also induced a specific immune response.
CONCLUSION: The successful construction of HP-NAP oral DNA vaccine with good immunogenicity may help to further investigate its immunoprotection effects and develop vaccine against H pylori infection.
-
Citation: Sun B, Li ZS, Tu ZX, Xu GM, Du YQ. Construction of an oral recombinant DNA vaccine from
H pylori neutrophil activating protein and its immunogenicity. World J Gastroenterol 2006; 12(43): 7042-7046 - URL: https://www.wjgnet.com/1007-9327/full/v12/i43/7042.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i43.7042
The discovery of H pylori has brought about a revolution in the research of etiological factors of gastrointestinal diseases[1]. It has been confirmed that H pylori is the main cause of chronic superficial gastritis, chronic active gastritis and peptic ulcer[2-4], and has a close relation to gastric mucosa-associated lymphoid tissue lymphoma and gastric cancer[5,6]. In 1994, the World Health Organization defined it as a class 1 carcinogen. Although significant progress has been made in treating H pylori infection with current triple or quadruple therapy based on antibiotics and proton pump inhibitors, the limitations of pharmacological therapy such as side effects, poor compliance, high cost, and most importantly, rapid emergence of antibiotic resistance have set the stage for the development of less costly and more efficient means to prevent and control H pylori infection. Ample precedence from previous experiences suggests that vaccination may be an alternative[7].
DNA vaccine has shown a great potential in protecting against and treating many diseases since it was developed. It can induce complete immune responses, provide heterologous cross protection, and can be easily prepared as a polyvalency vaccine[8]. In addition, the live attenuated Salmonella typhimurium (S. typhimurium) strain expressing foreign antigens may be a very hopeful new-generation for developing H pylori vaccine. Experiments on human body indicate that it has very good endurance and immunogenicity, which can be used to transmit foreign antigens[9]. In our present study, we selected a neutrophil activating protein (HP-NAP), a new major virulence factor of H pylori identified more recently, which was termed for its ability to induce adhesion of neutrophils to gastric endothelial cells and to produce reactive oxygen radicals[10]. We attempted to construct a live attenuated S. typhimurium strain harbouring the HP-NAP gene as an oral recombinant DNA vaccine, and to explore its immunogenicity to pave the way for biological treatment of H pylori infection.
The H pylori standard strain CCUG 17874, kindly presented by the IRIS Research Center of Italy, was cultured on Campylobacter selective agar (Merck, Germany) medium supplemented with 10% defibrillated goat blood containing Campylobacter selective antibiotic mixture (Merck), and incubated under microaerobic conditions (50 mL O2, 85 mL N2, 10 mL CO2 and 10% relative humidity at 37°C). The E. coli strain DH5α, live attenuated S. typhimurium strain LB5000 and SL7207, and COS-7 cell lines conserved in our laboratory, were cultured routinely.
Restriction enzymes including Xho I and Mlu I, T4 DNA ligase, and EX Taq™ DNA polymerase were purchased from TaKaRa Biotechnology (Dalian) Co., Ltd. Mouse anti-HP-NAP antibody was prepared by our laboratory. Alkaline phosphatase anti-mouse IgG made in horse was purchased from Vector Laboratories. Lipofectamine™ 2000 was purchased from Gibco Corporation. T-A cloning vector pBT was purchased from Shanghai Sangon Biological Engineering & Technologies and Services Co., Ltd. Eukaryotic expression vector pIRES was purchased from Clontech of BD Biosciences. Other reagents were analytically pure reagents produced in China.
Twenty-four specific-pathogen free male C57BL/6 mice aged 4 wk, were purchased from Sino-British Sippr/Bk Laboratory Animal Ltd. of Shanghai.
According to the nucleotide sequence of the HP-NAP gene in GenBank, we designed a pair of oligonucleotide primers P1 (5´-GTC CTC GAG ATG AAA ACA TTT GAA ATT TTA AAA CAT TTG CAA GCG-3', with Xho I restriction site) and P2 (5´-GTC ACG CGT TTA AGC CAA ATG GGC TTG CAA CAT CC-3', with Mlu I restriction site) synthesized by Sangon with correct ORF. Genomic DNA of CCUG 17874 was extracted as the template. Four mL of template DNA was added to a 100 mL reaction mixture containing 10 mL 10 × PCR buffer, 0.2 mmol/L each deoxynucleoside triphosphate, 2.5 U of EX Taq™ polymerase, and 0.2 mmol/L each primer. PCR was performed with Mastercycler® gradient thermocycler (Eppendorf, Germany) as follows. The initial denaturation cycle was at 94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 1 min, primer annealing at 60°C for 1 min, and DNA chain extension at 72°C for 1 min. The final cycle was at 72°C for 10 additional minutes, followed by rapid cooling to 4°C. The purified PCR products were T-A cloned into pBT vectors, then transformed into DH5α competent cells using standard methods. Positive clones were screened by blue/white spot and ampicilin resistance. Single-stranded DNA was prepared from selected clones for sequencing with forward M13 universal primer. Homologous analysis between the cloned HP-NAP gene and related genes in GenBank for neucleotide and deduced amino acid sequence was performed by BLAST. The identified recombinant plasmid pBT-NAP was digested with restriction endonucleases Xho I, Mlu I and subcloned into the corresponding sites of eukaryotic expression vector pIRES. PCR with primers P1, P2 and double enzyme restriction were performed.
COS-7 cells were cultured routinely and inoculated into 6-well plates one day before transfection, then co-cultured with a mixture of Lipofectamine™ 2000 and recombinant plasmid pIRES-NAP mixed instantly in definite proportion. After incubation at 37°C for 24-48 h, the culture was centrifuged to collect supernatant. Western blot analysis was performed to evaluate the immunity of HP-NAP antigen expressed in culture supernatant using mouse anti-HP-NAP as primary antibody and horse anti-mouse IgG as secondary antibody.
Recombinant plasmid pIRES-NAP was transformed into S. typhimurium strain LB5000 for methylation decoration, then extracted and transformed by electroporation into ending host bacteria S. typhimurium strain SL7207. SL7207 (pIRES-NAP) was grown in LB medium containing 100 mg/mL ampicilin at 37°C for 60 generations. Identification by PCR amplification and double restriction endonuclease digestion was performed every 10 generations.
Twenty-four mice were divided into 3 groups (8 mice in each group). LB group consisting of non-immunized mice received LB culture fluid and was used as a control group, Salmonella group was immunized merely with attenuated The S. typhimurium strain SL7207, the vaccine group was immunized with recombinant strain SL7207 (pIRES-NAP). Prior to immunization, all the mice were left overnight without solid food and 6 h without water. A total volume of 100 μL of 30 g/L sodium bicarbonate was given orally using a special catheter to neutralize the stomach pH. Then the mice in the LB group were lavaged immediately with 200 μL LB fluid. Mice in the Salmonella group and vaccine group were lavaged with 1.0 × 109 c.f.u of S. typhimurium strain SL7207 and vaccine strain SL7207 (pIRES-NAP), respectively, in a total volume of 200 μL. At 4 wk after immunization, the mice were sacrificed by terminal cardiac puncture under anesthesia and small intestine juices were collected. Indirect ELISA was performed to evaluate HP-NAP-specific IgG or IgA in serum and intestine juice. Purified HP-NAP was used as a coating antigen in ELISA immunoassay.
A 435 bp gene segment was amplified by PCR, which was consistent with the complete sequence of the HP-NAP gene confirmed by sequencing result (Figure 1). BLAST analysis showed that the nucleotide homology between cloned HP-NAP gene and H pylori SS1 strain from Genbank reached 98.2% (427/435), including 3 of C-T and 4 of A-G replacements, and more than 97% (426/435, 425/435) of other common H pylori strains such as 5D, 5A, 2B, 2A, RHP901a, DB2 and 1811a. Base replacements of cloned sequence did not significantly affect the translating results. The homology between proteins translated by cloned gene and SS1 strain was 98.6% (142/144).
A 435 bp target product was cloned as a template on the recombinant plasmid pIRES-NAP using primers P1 and P2. Xho I and Mlu I enzyme digestion also revealed the target HP-NAP gene in plasmid pIRES-NAP (Figure 2), suggesting that the recombinant plasmid pIRES-NAP and the oral DNA vaccine strain SL7207 (pIRES-NAP) were successfully constructed.
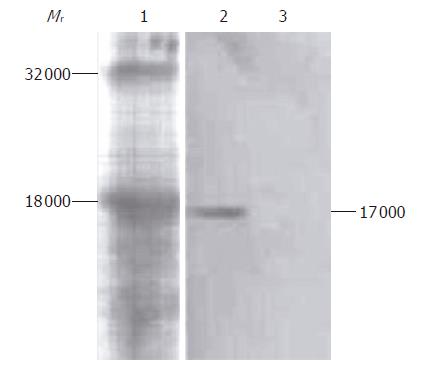
Western blot analysis results of culture supernatant of COS-7 cells transfected with recombinant plasmid pIRES-NAP are shown in Figure 3. A special simple strip about 17 000 in relative molecular weight was obtained from the supernatant of transfected COS-7 cells, corresponding to the presumed consequence, but no strip was found in the supernatant of non-transfected COS-7 cells as control.
The sera and intestinal juices of the vaccine group immunized with SL7207 (pIRES-NAP) showed positive ELISA results while those of the LB group and Salmonella group showed negative ELISA results, indicating that S. typhimurium SL7207 (pIRES-NAP) could enable the organism to generate specific mucosal and humoral immunity against the HP-NAP antigen.
Research on the H pylori vaccine has been mainly focused on the development of protein vaccines during the past decades, but the preparation and purification of protein antigens is time-consuming and laborious. Since effective immune responses rely on the presence of adjuvants, most of which are toxic to the organism, it is therefore important to develop new H pylori vaccines.
Recent advances in immunology and molecular biology have permitted development of DNA vaccines, which have a wide range of applications. Vaccines for such diseases as HIV infection, malaria, and tuberculosis are being developed by using plasmid DNA or viral or bacterial vectors to deliver the genes encoding antigens from pathogens to the host[11]. As live attenuated virus vaccines have come into being for decades, antigenic proteins can be produced in situ by the host, engendering cellular and humoral immune responses. But unlike live attenuated vaccines, gene-based vaccines are being designed to deliver only the genes encoding the antigens for the vaccine. The ability of gene-based vaccines to generate cellular as well as humoral responses may be crucial in developing effective vaccines against H pylori-induced diseases. Similarly, the ability of gene-based vaccines to generate certain forms of immunogen such as a protein with a particular structure that can be formed only by mammalian cells in situ, may be a critical feature of DNA vaccines. It is thought that DNA vaccines can be produced and distributed on a global scale for prevention of diseases such as HIV infection, malaria, and tuberculosis.
S. typhimurium can be phagocytized by macrophages and M cells in Peyer node and through mesentery lymph nodes reach the liver and spleen, further stimulating other organs and tissues to develop mucous membranes, cell and body fluid immunization responses. In the past few years, attenuated S. typhimurium as a delivery system has become a new trend to study a new type of oral recombinant live vaccine. Compared with traditional vaccines, live attenuated S. typhimurium is used as a new type of vector releasing system for heterologous antigens which does not require antigen purification, and not only protects antigens from degradation and denaturation in the stomach but also expresses adjuvant activity and prevents oral tolerance[12,13].
The development of H pylori DNA vaccine is unfolding. Todoroki et al[14] have investigated the effect of DNA vaccines encoding H pylori-heat shock proteins A and B (pcDNA3.1-hspA and -hspB) on inducing immune responses against H pylori in mice. In their study, C57BL/6 mice aged 5 wk were immunized by a single injection of 10 mg of pcDNA3.1-hspA and pcDNA3.1-hspB into intracutaneous tissue. Plasmid DNA lacking the inserted Hsp was injected as a control. Their results demonstrated that DNA vaccines encoding H pylori-Hsp could induce a significant immune response against H pylori and decrease gastric mucosal inflammation. Miyashita and his colleagues[15] reported that both intranasal and intracutaneous vaccination with pcDNA3.1 encoding H pylori-catalase (kat) induces humoral immune responses and suppresses H pylori colonization and inflammation of gastric mucosa. Serum IgG and IgA antibodies were induced in mice immunized with intracutaneous pcDNA3.1-kat with suppressed H pylori colonization compared to the mice immunized with control DNA, indicating that an effective DNA vaccine can be a new approach against H pylori infection in humans with potential foreground.
HP-NAP, a 150 000 dodecameric protein, is released in the medium, most likely after cell lysis, and binds to the bacterial surface, where it acts as an adhesion, mediating its binding to mucin[16] or to polymorphonuclear leukocyte sphingomyelin[17]. Purified recombinant HP-NAP is chemotactic for human neutrophils and monocytes[10] and induces surface expression of β2-integrins which are necessary for endothelial trans-migration[18], suggesting that HP-NAP plays a role in the accumulation of these cells at the H pylori-infected site. HP-NAP is also a powerful stimulant of the production of reactive oxygen radicals and acts via a cascade of intracellular activation events, including increase in cytosolic calcium ion concentration and phosphorylation of proteins, leading to the assembly of functional NADPH oxidase on neutrophil plasma membrane through a pertussis toxin-sensitive pathway involving extracellular-regulated kinase (ERK) and p38-mitogen-activated protein kinase (MAPK)[19]. The activation of ERK and p38-MAPK is essential for the HP-NAP-induced superoxide anion generation, adhesion and chemotaxis of human neutrophils. Cytokines such as TNF and IFN-γ are reported to enhance the production of ROIs induced by HP-NAP and increase the damage of gastric mucosa[20].
HP-NAP has also been shown to increase the synthesis of tissue factor and the secretion of inhibitor-2 of the plasminogen activator in mononuclear cells. By inducing the coordinate expression of cell pro-coagulant and antifibrinolytic activities, HP-NAP might favor fibrin deposition and contribute to the inflammatory reaction of gastric mucosa elicited by H pylori[21]. HP-NAP is also capable of crossing epithelial monolayers and inducing activation of the underlying mast cells[22]. These data further support the idea that HP-NAP has an important role in the in vivo triggering and maintaining of inflammatory events observed during H pylori infection. Once released from the bacterium, HP-NAP would traverse the stomach epithelial layer, reaching the underlying tissue where mast cells reside. The subsequent activation of mast cells by HP-NAP with release of the content of the granules and pro-inflammatory cytokine IL-6, is known to recruit monocytes and neutrophils. Thus, HP-NAP can act at different stages of the inflammatory response by activating mast cells with release of pro-inflammatory molecules able to activate neutrophils and monocytes, and additionally by acting directly on neutrophils and monocytes by promoting their recruitment and activation.
HP-NAP is highly immunogenic in humans. Analysis of serum samples from 35 H pylori-infected individuals revealed that 60% of the subjects contain specific antibodies to HP-NAP[2]. Satin and his colleagues[10] immunized 10 mice with purified recombinant HP-NAP by intragastric administration, showing that 80% of them have acquired protective immunity, which is higher than the CagA (70%) group but lower than the supersonic lysate group (90%). These data indicate that this protein is a good vaccine candidate for protecting H pylori infection.
In the present study, we successfully amplified and subcloned the HP-NAP gene into the eukaryotic expression vector pIRES, and established the recombinant live attenuated S. typhimurium strain SL7207 (pIRES-NAP) as an oral DNA vaccine. BLAST analysis indicated the HP-NAP gene we cloned had a high homology with those in GenBank. The COS-7 cells transfected with recombinant plasmid pIRES-NAP expressing a protein of 17 000 reacted especially with anti-HP-NAP antibody, but no reaction was found in the control group. On the other hand, the serum and intestine fluids from mice immunized with DNA vaccine contained specific antibody to HP-NAP, while those from control groups did not.
In conclusion, HP-NAP DNA vaccine can express target protein with good immunogenicity in eukaryotic hosts, and S. typhimurium strain SL7207 (pIRES-NAP) may be a good candidate as a vaccine for prevention and cure of H pylori infection. Further study is needed to explore its immunoprotection effects.
S- Editor Wang J L- Editor Wang XL E- Editor Bi L
| 1. | Kirsner JB. The origin of 20th century discoveries transforming clinical gastroenterology. Am J Gastroenterol. 1998;93:862-871. [PubMed] |
| 2. | Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113:321-333. [PubMed] |
| 3. | Sanders MK, Peura DA. Helicobacter pylori-Associated Diseases. Curr Gastroenterol Rep. 2002;4:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Olbe L, Fandriks L, Hamlet A, Svennerholm AM, Thoreson AC. Mechanisms involved in Helicobacter pylori induced duodenal ulcer disease: an overview. World J Gastroenterol. 2000;6:619-623. [PubMed] |
| 5. | Sun B, Yang H, Tu ZX, Li ZS. Helicobacter pylori and gastric cancer. Acad J Sec Mil Med Univ. 2003;24:1356-1358. |
| 6. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3183] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 7. | Jiang Z, Huang AL, Tao XH, Wang PL. Construction and characterization of bivalent vaccine candidate expressing HspA and M(r)18,000 OMP from Helicobacter pylori. World J Gastroenterol. 2003;9:1756-1761. [PubMed] |
| 8. | Liu MA. DNA vaccines: a review. J Intern Med. 2003;253:402-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 229] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 9. | Del Giudice G, Covacci A, Telford JL, Montecucco C, Rappuoli R. The design of vaccines against Helicobacter pylori and their development. Annu Rev Immunol. 2001;19:523-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 165] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Satin B, Del Giudice G, Della Bianca V, Dusi S, Laudanna C, Tonello F, Kelleher D, Rappuoli R, Montecucco C, Rossi F. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J Exp Med. 2000;191:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 237] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 11. | Srivastava IK, Liu MA. Gene vaccines. Ann Intern Med. 2003;138:550-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Gentschev I, Dietrich G, Spreng S, Kolb-Mäurer A, Brinkmann V, Grode L, Hess J, Kaufmann SH, Goebel W. Recombinant attenuated bacteria for the delivery of subunit vaccines. Vaccine. 2001;19:2621-2628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Dietrich G, Kolb-Mäurer A, Spreng S, Schartl M, Goebel W, Gentschev I. Gram-positive and Gram-negative bacteria as carrier systems for DNA vaccines. Vaccine. 2001;19:2506-2512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Todoroki I, Joh T, Watanabe K, Miyashita M, Seno K, Nomura T, Ohara H, Yokoyama Y, Tochikubo K, Itoh M. Suppressive effects of DNA vaccines encoding heat shock protein on Helicobacter pylori-induced gastritis in mice. Biochem Biophys Res Commun. 2000;277:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Miyashita M, Joh T, Watanabe K, Todoroki I, Seno K, Ohara H, Nomura T, Miyata M, Kasugai K, Tochikubo K. Immune responses in mice to intranasal and intracutaneous administration of a DNA vaccine encoding Helicobacter pylori-catalase. Vaccine. 2002;20:2336-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Namavar F, Sparrius M, Veerman EC, Appelmelk BJ, Vandenbroucke-Grauls CM. Neutrophil-activating protein mediates adhesion of Helicobacter pylori to sulfated carbohydrates on high-molecular-weight salivary mucin. Infect Immun. 1998;66:444-447. [PubMed] |
| 17. | Teneberg S, Miller-Podraza H, Lampert HC, Evans DJ Jr, Evans DG, Danielsson D, Karlsson KA. Carbohydrate binding specificity of the neutrophil-activating protein of Helicobacter pylori. J Biol Chem. 1997;272:19067-19071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Evans DJ, Evans DG Jr, Takemura T, Nakano H, Lampert HC, Graham DY, Granger DN, Kvietys PR. Characterization of a Helicobacter pylori neutrophil-activating protein. Infect Immun. 1995;63:2213-2220. [PubMed] |
| 19. | Nishioka H, Baesso I, Semenzato G, Trentin L, Rappuoli R, Del Giudice G, Montecucco C. The neutrophil-activating protein of Helicobacter pylori (HP-NAP) activates the MAPK pathway in human neutrophils. Eur J Immunol. 2003;33:840-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Dundon WG, Nishioka H, Polenghi A, Papinutto E, Zanotti G, Montemurro P, Del GG, Rappuoli R, Montecucco C. The neutrophil-activating protein of Helicobacter pylori. Int J Med Microbiol. 2002;291:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Montemurro P, Barbuti G, Dundon WG, Del Giudice G, Rappuoli R, Colucci M, De Rinaldis P, Montecucco C, Semeraro N, Papini E. Helicobacter pylori neutrophil-activating protein stimulates tissue factor and plasminogen activator inhibitor-2 production by human blood mononuclear cells. J Infect Dis. 2001;183:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Montemurro P, Nishioka H, Dundon WG, de Bernard M, Del Giudice G, Rappuoli R, Montecucco C. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a potent stimulant of mast cells. Eur J Immunol. 2002;32:671-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |