Published online Nov 21, 2006. doi: 10.3748/wjg.v12.i43.6941
Revised: September 15, 2006
Accepted: September 20, 2006
Published online: November 21, 2006
Since the relationship between angiogenesis and tumor growth was established by Folkman in 1971, scientists have made efforts exploring the possibilities in treating cancer by targeting angiogenesis. Inhibition of angiogenesis growth factors and administration of angiogenesis inhibitors are the basics of anti-angiogenesis therapy. Transfer of anti-angiogenesis genes has received attention recently not only because of the advancement of recombinant vectors, but also because of the localized and sustained expression of therapeutic gene product inside the tumor after gene transfer. This review provides the up-to-date information about the strategies and the vectors studied in the field of anti-angiogenesis cancer gene therapy.
- Citation: Liu CC, Shen Z, Kung HF, Lin MC. Cancer gene therapy targeting angiogenesis: An updated Review. World J Gastroenterol 2006; 12(43): 6941-6948
- URL: https://www.wjgnet.com/1007-9327/full/v12/i43/6941.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i43.6941
Angiogenesis is the formation of new blood vessels from pre-existing ones. Many developmental and pathological processes require angiogenesis[1]. As proposed by Folkman in 1971, angiogenesis is required for tumor growth[2]. Angiogenesis consists of several steps: endothelial cell (EC) proliferation, migration, basement membrane degradation, and new lumen organization[3]. This multi-step process is determined by a net balance between pro- and anti-angiogenesis regulators in the circulation blood, which are released from activated ECs, monocytes, smooth muscle cells and platelets[3].
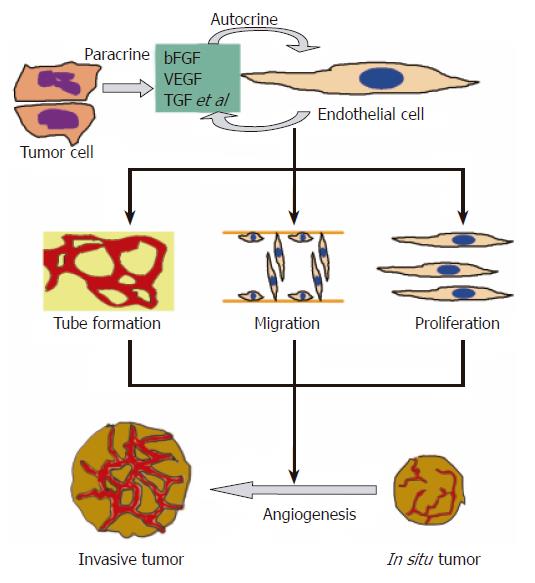
The growth of tumor depends on new blood vessel growth and involves three steps: angiogenesis, vasculogenesis and intussusception[4]. Without angiogenesis, a solid tumor rarely grows larger than 2 to 3 mm[5]. As shown in Figure 1, ECs and tumor cells release angiogenesis regulators like vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and transforming growth factor (TGF) to mediate angiogenesis. The result is the development of invasive tumor. In addition to the presence of angiogenesis factors, activation of oncogene and loss of tumor suppressor gene are also essential for an angiogenesis phenotype that supports tumorigenicity[6]. As a result, anti-angiogenesis has been regarded as a target for cancer therapy.
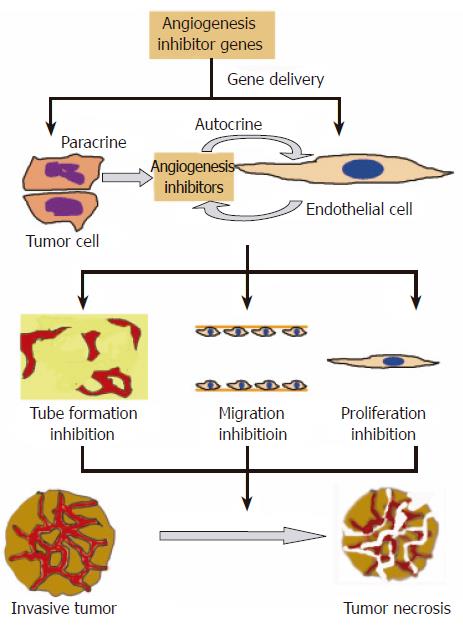
There are already several extensive reviews on the development of anti-angiogenesis cancer gene therapy[3,7-10]. In the 2001 review, Liau et al[7] compared and contrasted the gene approach and recombinant protein approach. In the editorial written by Lau and Bicknel[9], the authors compared the delivery of the genes of anti-angiogenic factors with that of the therapeutic proteins. They suggested that the delivery of genes can allow a high local expression of the protein at the sites of active tumor growth[9]. El-Aneed pointed out in his review, which summarizes the strategies in cancer gene therapy, that the ease of accessing ECs of the blood vessels is one of the main advantages of gene delivery approach[10]. Figure 2 shows that the delivery of the anti-angiogenesis gene into tumor cells or ECs can inhibit tube formation, EC migration and proliferation. This can result in tumor necrosis. In this review, updated information on the development of cancer anti-angiogenesis gene therapy is discussed.
RNA interference (RNAi) is the sequence-specific gene silencing induced by double-stranded RNA. Introduction of 21-23 small interfering RNAs (siRNAs) of the nucleotide can knock-out the expression of a particular gene[11]. A recent review written by Izquierdo[12] pointed out that siRNA against expression of vascular growth factor receptor (VGFR) can reduce tumor volume by blocking angiogenesis. Kwon and co-workers[13] described a method that can suppress the expression of VGFR-A at both transcriptional and post-transcriptional levels by a combination of zinc finger protein and siRNA. Gondi and co-workers[14] have demonstrated the potential application of RNAi in gene cancer therapy by inhibiting angiogenesis in both in vivo and in vitro human glioma cell models. Furthermore, it is possible to include more than one antiangiogenesis siRNA into a single retroviral vector because of the small size of siRNA, which could inhibit multiple pathways[12].
Antisense oligodeoxynucleotide (ODN) is a synthetic molecule that blocks mRNA translation. The blockade of translation of mRNA of pro-angiogenesis factor genes can result in inhibition of tumor growth. Recently, ODN blocking of the expression of VEGF has been shown to be a promising cancer gene therapy. For example, Wang et al[15] have successfully reduced VEGF protein expression by 45% in human osteosarcoma cell line by transducing a eukaryotic expression plasmid containing antisense VEGF. Lipiodol is an effective treatment for unresectable liver cancer through transcatheter arterial embolization of the hepatic artery[16]. When VEGF antisense ODN is mixed with lipiodol, this combinational approach is better in inhibiting liver cancer growth, VEGF expression and microvessel density[16].
Table 1 summarizes the genes of candidate angiogenesis inhibitors that have been studied recently. In the review published in the Journal of Translational Medicine[3], the authors made a thorough account of several candidates. To avoid overlapping of information, we only discuss those candidates that are not covered or recently have demonstrated significant advancement.
| Candidate | Reference(s) |
| 16 kD prolactin fragment1 | 3 |
| Angiostatin1 | 3, 36 |
| Arresten1 | 3 |
| Canstatin1 | 3 |
| Endostatin1 | 3 |
| Endothelial-monocyte activating polypeptide-II (EMAP-II)1 | 3 |
| Fragments of hepatocyte growth factor (HGF) | |
| NK4 | 40, 41 |
| HGFK1 | 42 |
| Human rubonuclease inhibitor (hRI) | 20, 21 |
| Interferon-inducible protein-10 (IP-10)1 | 3 |
| Interferons1 | 3 |
| Interleukin-12 (IL-12)1 | 28, 29 |
| Interleukin-18 (IL-18)1 | 3 |
| Interleukin-24 (IL-24) | 37, 38, 39 |
| Maspin | 17, 18, 19 |
| p531 | 3 |
| Pigment epithelium-derived factor (PEDF) | 31, 32, 33, 34 |
| Platelet factor-41 | 3 |
| Restin1 | 3 |
| Soluble FMS-like tyrosine kinase receptor 1 (sFlt-1) | 26, 27 |
| Survivin | 22, 23, 24, 25 |
| Thrombospondin-1 (THBS1)1 | 3 |
| Tissue inhibitors of metalloproteinases (TIMPs)1 | 3, 35 |
| Tumor necrosis factor alpha (TNF-α)1 | 3 |
| Tumstatin1 | 3 |
| Vastatin1 | 43 |
Maspin: The Maspin gene is a tumor suppressor gene which is under transcriptional control by p35 and DNA methyltransferase inhibitors. Its gene expression level decreases with malignancy and is lost in metastatic cells[17,18]. Transfection of maspin gene to nude mice could reduce the ability of cells to induce tumors and metastasis[19]. Recently, Watanabe et al[18] have shown that adeno-associated virus 2-mediated expression of human maspin can efficiently suppress tumor growth by inhibition of angiogenesis in prostate cancer.
Human ribonuclease inhibitor: Human ribonuclease inhibitor (hRI) is an acidic protein with a molecular weight of 50 kDa. It can inhibit the activity of pancreatic RNase (RNase A)[20]. It is proposed that hRI inhibits angiogenesis by forming a tight complex with its counterpart angiogenin (Ang) which is an angiogenesis factor[21]. Fu et al[20] demonstrated that hematopoietic cells carrying the ri gene can effectively inhibit tumor growth (by 47%) and reduce tumor microvessel density in mice. They concluded that hRI has the potential utility as a novel antiangiogenesis agent[20].
Survivin: Survivin has been identified as an anti-apoptosis gene over-expressed in cancer and lymphoma[22]. It has been shown that survivin is minimally expressed in endothelium of non-proliferating capillaries of normal skin, whereas it becomes massively up-regulated in newly formed blood vessels of granulation tissue in vivo. As a result, manipulation of survivin expression and function in endothelium may influence tumor angiogenesis[23]. Recently, a DNA vaccine targeting survivin and an adeno-associated viral vector carrying survivin Cys84Ala mutant have been employed to demonstrate the anti-angiogenesis effect on lung tumor and colon cancer cells respectively[24,25].
Soluble FMS-like tyrosine kinase receptor 1: Soluble FMS-like tyrosine kinase receptor 1 (sFlt-1) has been identified as a receptor of vascular endothelial growth factor (VEGF)[26]. It functions by sequestering VEGF and forming inactive heterodimers with other membrane-spanning VEGF receptors both in in vitro and in vivo[26]. Intramuscular injection of recombinant adeno-associated virus (rAAV) vectors carrying the sFlt-1 gene into nude mice can protect against the human ovarian cancer cell line with increased disease-free survival[27].
Interleukin-12: In 2004 and 2005, Heinzerling et al[28] and Imagawa et al[29] showed that direct intratumoral injection of interleukin-12 (IL-12) gene produces a reduction in vessel density or angiogenesis in a murine head and neck cancer model and in patients with metastatic melanoma[28,29]. It is worth noting that a few cytokines, like IL-12, have recently been reported to participate in the regulation of the angiogenic switch[30]. These cytokines are related to inflammation. Whether there are relationships between angiogenesis and inflammation may be an interesting topic among scientists in the future.
Pigment epithelium-derived factor: Pigment epithelium-derived factor (PEDF) is a neurotrophic protein and belongs to the serine protease inhibitor (serpin) family[31,32]. It is believed to be a potent inhibitor of angiogenesis[33]. A full-length human PEDF expression vector has been used to transfect the glioma cell line U251, resulting in up-regulation and down-regulation of angiogenesis inhibitors and activators[34].
Tissue inhibitors of metalloproteinase: In 2004, adeno-associated virus-mediated gene transfer of tissue inhibitor of metalloproteinase (TIMP) to animal’s tumor also showed that it can inhibit vascular tumor growth and angiogenesis[35].
Angiostatin: Angiostatin is a 38 kD kringle domain of plasminogen and is the most potent and well characterized body’s angiogenesis inhibitor[3]. Recently, the effectiveness of co-administration of the mouse angiostatin kringle and the endostatin genes using cationic liposome has been investigated in vitro and in vivo by Kim et al[36].
Melanoma differentiation-associated-7 gene or interleukin-24: Melanoma differentiation-associated-7 (mda-7) gene is a novel melanoma differentiation-associated gene that modulates human melanoma differentiation, growth and progression. It was identified by subtractive hybridization in human melanoma cells by Jiang et al[37]. It has been demonstrated that the mda-7 gene functions as a multi-modality anti-cancer agent, possessing both pro-apoptotic and anti-angiogenic properties, and the adenovirus-mediated over-expression of mda-7 gene has the potential therapeutic effects in human lung cancer[38]. More recently, Nishikawa et al[39] performed a combination therapy on non-small-cell lung cancer (NSCLC) cell lines and showed that the combination of mda-7 gene therapy and radiotherapy may be a feasible and effective strategy for treatment of NSCLC.
Fragments of hepatocyte growth factor: NK4 is the N-terminal hairpin domain and subsequent four-kringle domains of hepatocyte growth factor (HGF). It was reported that HGF possesses anti-angiogenesis property[40]. A latest trial has been done using hydrodynamics-based gene delivery of naked NK4 plasmid into colon cancer cells in mice. HGF can efficiently express NK4, inhibit liver metastasis and subsequent invasive growth of colon cancer and prolong survival of mice[41]. In addition to NK4, recombinant kringle 1 domain of HGF (HGFK1) has been shown to inhibit bovine aortic endothelial cell proliferation stimulated by basic fibroblast growth factor (bFGF) in a dose-dependent manner[42]. These studies present the potency of the fragments of HGF in inhibiting angiogenesis.
NC1 domains of collagen: Endostatin (from collagen XVIII), restin (from collagen XV), arrestin (α 1 chain of collagen IV) and canstatin (α 2 chain of collagen IV) are all NC1 domains as reviewed by Tandle et al[3]. Recently it has been shown that vastatin, the NCI domain of collagen VIII (α 1) possesses anti-angiogenesis ability in bovine aortic endothelial cells[43]. This provides another promising candidate for caner anti-angiogenesis gene therapy.
The viral vectors used for tumor vascular targeting therapy are summarized in a recent review[44]. Tandle et al[3] have also discussed some non-viral gene delivery vectors. Again, we will focus on those newly studied viral vectors showing advancement. Table 2 summarizes the vectors that are recently used in cancer antiangiogenesis gene therapy.
| Vector | Brief description | Reference (s) |
| Cationic liposomes | Spherical vesicle made of positively charged lipids, efficient uptake of DNA by the cell | 46, 47 |
| Low Voltage Electroporation | Entry of DNA into the cell whose membrane is permeabilized by electric field, efficient gene transfer is ensured | 48, 49 |
| Nanoparticles | Submicron-sized particle with the therapeutic agent situated within the matrix or on the surface | 3, 45 |
| Measles virus | Contains negative strand RNA molecule, can enter tumor cells without the defensive responses of the tumor | 44 |
| Herpes simplex virus | Dobule stranded DNA virus, wide host range, large transgene capacity, long-lasting effect | 3, 44 |
| Lentivirus | Derived from HIV, can achieve stable integration of the gene in non-dividing cells | 3, 44 |
| Retrovirus | Based on murine leukemia virus, foreign cDNA can be incorporated into host cell genome with high efficiency | 3, 44 |
| Replication-competent Retrovirus (RCR) | Modified retroviral vector that can replicate in solid tumor model so the transfer efficiency is enhanced | 55, 56 |
| Semliki Forest Virus (SFV) | One type of alphavirus, modification of its RNA genome yield a new expression vector that transfers transgene into tumor | 50, 51, 52, 53, 54 |
| Recombinant adenovirus (rAdv) | Double stranded DNA virus, can be produced in high titres and deliver transgene efficiently Special engineered adenovirus: oncolytic and gutless | 3, 57, 58, 59, 60, 61, 62, 63, 64 |
| Recombinant adeno-associated virus (rAAV) | Possess the advantages of rAdv and retrovirus, low level of immune response | 3, 25, 65, 66, 67, 68 |
Nanoparticles: Polymeric drug carriers are used to deliver low molecular mass drugs, oligonucleotides and peptides, which has attracted attention in recent years[3]. Due to their small sizes, nanoparticles penetrate into even small capillaries and are taken up by cells that can deliver targeted drugs to cells or tissues[3]. In 2005, Schiffelers et al[45] constructed self-assembling nanoparticles with siRNA as a means to target tumor neovasculature expressing integrins and to deliver siRNA which inhibits VEGF-R2 expression and thereby tumor angiogenesis. They pointed out that this mode of delivery overcomes the pharmacological hurdles of local administration of aqueous siRNA for cancer therapy.
Cationic liposome: The advantages of using a cationic liposome as a vehicle for drug delivery are the enhancement of delivery and expression of the transfected gene. The positive charge significantly increases the uptake of liposome by the endothelial cells of blood vessels in tumor tissues, which has made the cationic liposome useful for delivering tumor targeted drugs[46]. A recent successful case of angiogenesis inhibition using angiostatin and endostatin genes delivered by a cationic liposome has been reported. In addition, modified liposome targeting membrane type-1 matrix metalloproteinase (MT1-MMP) molecules expressed specifically on angiogenesis endothelium and tumor cells, enhances its binding to and accumulates ECs in tumor compared to unmodified liposome[47].
Low voltage electroporation: Electroporation is the formation of pores on the cell surface induced by electric pulse. Direct delivery of plasmid DNA into cells relies on electroporation. In vivo electroporation is a novel non-viral means of gene transfer and offers several advantages over viral means such as none of immunogenicity, ease of handling and high gene transfer efficiency[48]. Uesato and co-workers[49] have successfully demonstrated the anti-tumor effect of antiangiogenesis genes, mouse angiostatin and mouse endostatin, delivered to tumors by low-voltage electroporation in 26 models of mouse colon. They have also reported a decrease in microvessel density of tumors[49].
Semliki forest virus: A new expression vector system derived from semiliki forest virus (SFV) was introduced in 1994[50]. This system has been utilized in delivering glycoproteins in a recombinant vaccine study[51]. The vector has also been shown to be a candidate medium for human cancer gene therapy[52]. More recently, SFV vector carrying murine IL-12 gene demonstrated by Doppler ultrasonography, could cause B16 tumor regression through anti-angiogenesis[53]. After this, two IL-12 gene subunits cloned from mouse splenocytes and inserted into an enhanced SFV vector (pSFV10-E) could show complete tumor regression in mice[54].
Replication-competent retroviruses: Retroviruses are a class of virus which has a genome of a single stranded RNA molecule. Vectors derived from murine leukemia virus, a simple retrovirus, have been used in in vivo gene transfer in gene therapy. However, the limited efficiency of replication-defective retrovirus vector is a major obstacle in cancer gene therapy[55]. Logg’s group in Los Angeles thus developed a replication-competent retrovirus (RCR) vector derived from murine leukemia virus[55]. This vector is able to replicate and transmit a transgene both in culture and in a solid tumor model in vivo. By taking advantages from RCR vectors, Sun et al[56] transduced RCR vectors carrying the human interferon-inducible protein-10 (IP-10) gene to tumor cells in vivo and in vitro, showing sustained production of IP-10 in culture and reduced angiogenesis in mice.
Recombinant adenovirus: Adenovirus has a double stranded DNA genome. Recombinant adenovirus (rAdv) vectors containing exogenous genes for in vivo transfer derived from adenovirus type 5 are made replication deficient by deletion of the E1 region[57]. rAdv is currently the most widely used gene delivery vector because it enjoys several advantages like high delivery efficiency into both dividing and non-dividing cells, large ability to package foreign genes, easy to grow to high titers and to be purified, non-oncogenic and high expression of the transgenes[58]. In recent years, phaseItrials have been undertaken using adenoviral p53 (Adp53) for patients with ovarian cancer[59]. In China, phaseIand II trials using recombinant Adp53 to treat laryngeal cancer (phaseI), head and neck squamous cell carcinoma (phase II) and nasopharyngeal carcinoma (phase II) have been undertaken extensively[60-62].
Oncolytic adenovirus is a specially engineered adenovirus which exhibits lytic property of virus replication[63]. This adenoviral system not only offers the advantage of high gene delivery efficiency, but also the ability to select infections of tumor cells[63]. As a result, an amplification effect of the therapeutic gene can be achieved through the lateral spread of the progeny vector[63].
The latest generation of adenoviral vector is the gutless adenovirus. It has become an attractive agent for gene therapy because of the reduction of in vivo immune response[64] and long-term sustained expression. However, because of the lack of all viral coding regions, the packaging of this virus requires the presence of helper virus which presents the possibility of contamination[64].
Recombinant adeno-associated virus: Recombinant adeno-associated virus (rAAV) has the advantages of broad host range, low level of immune response, and longevity of gene expression that enable the initiation of a number of clinical trials using this gene delivery system[65]. As reviewed recently, there are 8 well-defined serotypes (serotypes 1-5 and 7-9), and more than 100 variants[66]. The underlying mechanism of the selective tissue tropism of different serotypes remains elusive[66]. For anti-angiogenesis cancer gene therapy using rAAV, recent research examples are focusing on treating colon cancer (in vitro and in vivo), ovarian cancer (in vivo) and human glioblastoma (in vitro)[25,67,68].
While previous studies on gene targets are limited to pre-clinical stages, the recombinant proteins of some of these targets have entered clinical trials. Can we learn lessons from the trials to optimize the specificity and efficiency of the candidate gene therapeutics
Recombinant endostatin is currently the most studied angiogenesis inhibitor in the clinical setting. The earliest phaseItrials were published in 2002 and 2003[69-72]. However, the results were disappointing. Two very recent reports stated that although the endostatin trials have confirmed the safety of endostatin as a pharmacological agent, it is difficult to establish the biologically effective dose of the recombinant protein[73,74]. To address the problem of effective dose of endostatin, Tjin Tham Sjin et al[75] recently demonstrated that adeno-associated viruses carrying canine endostatin can dose-dependently express transgene in the circulation after intramuscular injection in mice. Elevated levels of endostatin remain stable in the circulation for at least 4 mo[75]. Therefore, adeno-associated virus-mediated endostatin gene therapy appears to be a potential therapeutic regime with specific and sustained delivery efficiency.
IL-12 is another widely studied agent with anti-angiogenesis activity in clinical trials. Recombinant human IL-12 protein has entered phaseIand II studies in Germany and United States, respectively[3]. Due to the occurrence of dose-limiting toxicity in some patients, the direction of study has switched to gene therapy approaches[3]. A phaseItrial involving an adenoviral vector encoding human IL-12 gene has been conducted[76], showing that dose-limited toxicity is significantly increased in tumor infiltration by effector immune cells. Despite the lower anti-tumor power of IL-12 gene therapy in human trials, the concept of stimulation of immune response by specific production of IL-12 inside a tumor is proved[77].
Recently, attention has been paid to combination therapy in which anti-angiogenesis treatment is combined with chemotherapy as well as radiotherapy[78]. Approaches like combination of endostatin and VGFR-2 tyrosine kinase inhibitor and even tri-combination of anti-angiogenesis, chemotherapy and radiotherapy have also been tested[79,80]. Co-targeting of tumor and tumor micro-environment can effectively suppress angiogenesis and tumor growth in the prostate cancer model[81]. A Chinese phase III trial using recombinant endostatin in combination with chemotherapy in NSCLC has exhibited a significant increase in response rates and time to progression[73].
Specificity and safety of the vectors are the two main issues that should be addressed in the future. Development of vectors that exhibit superior safety and direct the therapeutic transgene to the right target position of the genome without any random insertion side effects would be a direction for studying human gene therapy against cancer.
Targeting angiogenesis is a promising approach in suppressing tumor growth and metastasis. Due to the need for long term administration of the inhibitors, gene therapy has become an alternative which theoretically ensures a sustained availability of the anti-angiogenesis agents. Up till now, researches on anti-angiogenesis cancer gene therapy remain in pre-clinical stage. It is anticipated that when better vectors are developed and the molecular mechanisms of angiogenesis inhibitors against tumor growth are better understood, clinical trials will be undertaken in the future.
S- Editor Wang J L- Editor Wang XL E- Editor Liu WF
| 1. | Cavallaro U, Christofori G. Molecular mechanisms of tumor angiogenesis and tumor progression. J Neurooncol. 2000;50:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5115] [Cited by in RCA: 5900] [Article Influence: 109.3] [Reference Citation Analysis (1)] |
| 3. | Tandle A, Blazer DG 3rd, Libutti SK. Antiangiogenic gene therapy of cancer: recent developments. J Transl Med. 2004;2:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Cao Y. Tumor angiogenesis and therapy. Biomed Pharmacother. 2005;59 Suppl 2:S340-S343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5621] [Cited by in RCA: 5531] [Article Influence: 184.4] [Reference Citation Analysis (0)] |
| 6. | Volpert OV, Dameron KM, Bouck N. Sequential development of an angiogenic phenotype by human fibroblasts progressing to tumorigenicity. Oncogene. 1997;14:1495-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 135] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Liau G, Su EJ, Dixon KD. Clinical efforts to modulate angiogenesis in the adult: gene therapy versus conventional approaches. Drug Discov Today. 2001;6:689-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Chen QR, Zhang L, Gasper W, Mixson AJ. Targeting tumor angiogenesis with gene therapy. Mol Genet Metab. 2001;74:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Lau K, Bicknell R. Antiangiogenic gene therapy. Gene Ther. 1999;6:1793-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | El-Aneed A. Current strategies in cancer gene therapy. Eur J Pharmacol. 2004;498:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Campbell TN, Choy FY. RNA interference: past, present and future. Curr Issues Mol Biol. 2005;7:1-6. [PubMed] |
| 12. | Izquierdo M. Short interfering RNAs as a tool for cancer gene therapy. Cancer Gene Ther. 2005;12:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Kwon HS, Shin HC, Kim JS. Suppression of vascular endothelial growth factor expression at the transcriptional and post-transcriptional levels. Nucleic Acids Res. 2005;33:e74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Gondi CS, Lakka SS, Dinh DH, Olivero WC, Gujrati M, Rao JS. RNAi-mediated inhibition of cathepsin B and uPAR leads to decreased cell invasion, angiogenesis and tumor growth in gliomas. Oncogene. 2004;23:8486-8496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Wang Y, Wang R, Qiao H, Li JY, Peng TS, Li Y, Zhang M, Liang HZ, Qiu JS. [Targeted inhibition of vascular endothelial growth factor (VEGF) expression in human osteosarcoma cell line by antisense VEGF165 cDNA promoted by hypoxia reaction element]. Zhonghua Binglixue Zazhi. 2005;34:588-591. [PubMed] |
| 16. | Wu HP, Feng GS, Liang HM, Zheng CS, Li X. Vascular endothelial growth factor antisense oligodeoxynucleotides with lipiodol in arterial embolization of liver cancer in rats. World J Gastroenterol. 2004;10:813-818. [PubMed] |
| 17. | Zhang M, Maass N, Magit D, Sager R. Transactivation through Ets and Ap1 transcription sites determines the expression of the tumor-suppressing gene maspin. Cell Growth Differ. 1997;8:179-186. [PubMed] |
| 18. | Watanabe M, Nasu Y, Kashiwakura Y, Kusumi N, Tamayose K, Nagai A, Sasano T, Shimada T, Daida H, Kumon H. Adeno-associated virus 2-mediated intratumoral prostate cancer gene therapy: long-term maspin expression efficiently suppresses tumor growth. Hum Gene Ther. 2005;16:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, Rafidi K, Seftor E, Sager R. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 689] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 20. | Fu P, Chen J, Tian Y, Watkins T, Cui X, Zhao B. Anti-tumor effect of hematopoietic cells carrying the gene of ribonuclease inhibitor. Cancer Gene Ther. 2005;12:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Kurachi K, Davie EW, Strydom DJ, Riordan JF, Vallee BL. Sequence of the cDNA and gene for angiogenin, a human angiogenesis factor. Biochemistry. 1985;24:5494-5499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 192] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2342] [Cited by in RCA: 2383] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 23. | O'Connor DS, Schechner JS, Adida C, Mesri M, Rothermel AL, Li F, Nath AK, Pober JS, Altieri DC. Control of apoptosis during angiogenesis by survivin expression in endothelial cells. Am J Pathol. 2000;156:393-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 259] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 24. | Xiang R, Mizutani N, Luo Y, Chiodoni C, Zhou H, Mizutani M, Ba Y, Becker JC, Reisfeld RA. A DNA vaccine targeting survivin combines apoptosis with suppression of angiogenesis in lung tumor eradication. Cancer Res. 2005;65:553-561. [PubMed] |
| 25. | Tu SP, Cui JT, Liston P, Huajiang X, Xu R, Lin MC, Zhu YB, Zou B, Ng SS, Jiang SH. Gene therapy for colon cancer by adeno-associated viral vector-mediated transfer of survivin Cys84Ala mutant. Gastroenterology. 2005;128:361-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Kendall RL, Wang G, Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun. 1996;226:324-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 517] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 27. | Mahendra G, Kumar S, Isayeva T, Mahasreshti PJ, Curiel DT, Stockardt CR, Grizzle WE, Alapati V, Singh R, Siegal GP. Antiangiogenic cancer gene therapy by adeno-associated virus 2-mediated stable expression of the soluble FMS-like tyrosine kinase-1 receptor. Cancer Gene Ther. 2005;12:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Imagawa Y, Satake K, Kato Y, Tahara H, Tsukuda M. Antitumor and antiangiogenic effects of interleukin 12 gene therapy in murine head and neck carcinoma model. Auris Nasus Larynx. 2004;31:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Heinzerling L, Burg G, Dummer R, Maier T, Oberholzer PA, Schultz J, Elzaouk L, Pavlovic J, Moelling K. Intratumoral injection of DNA encoding human interleukin 12 into patients with metastatic melanoma: clinical efficacy. Hum Gene Ther. 2005;16:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Cao R, Farnebo J, Kurimoto M, Cao Y. Interleukin-18 acts as an angiogenesis and tumor suppressor. FASEB J. 1999;13:2195-2202. [PubMed] |
| 31. | Becerra SP, Palmer I, Kumar A, Steele F, Shiloach J, Notario V, Chader GJ. Overexpression of fetal human pigment epithelium-derived factor in Escherichia coli. A functionally active neurotrophic factor. J Biol Chem. 1993;268:23148-23156. [PubMed] |
| 32. | Steele FR, Chader GJ, Johnson LV, Tombran-Tink J. Pigment epithelium-derived factor: neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc Natl Acad Sci USA. 1993;90:1526-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 336] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 33. | Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, Bouck NP. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1069] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 34. | Guan M, Pang CP, Yam HF, Cheung KF, Liu WW, Lu Y. Inhibition of glioma invasion by overexpression of pigment epithelium-derived factor. Cancer Gene Ther. 2004;11:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Zacchigna S, Zentilin L, Morini M, Dell'Eva R, Noonan DM, Albini A, Giacca M. AAV-mediated gene transfer of tissue inhibitor of metalloproteinases-1 inhibits vascular tumor growth and angiogenesis in vivo. Cancer Gene Ther. 2004;11:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Kim KS, Kim HS, Park JS, Kwon YG, Park YS. Inhibition of B16BL6 tumor progression by coadministration of recombinant angiostatin K1-3 and endostatin genes with cationic liposomes. Cancer Gene Ther. 2004;11:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11:2477-2486. [PubMed] |
| 38. | Saeki T, Mhashilkar A, Swanson X, Zou-Yang XH, Sieger K, Kawabe S, Branch CD, Zumstein L, Meyn RE, Roth JA. Inhibition of human lung cancer growth following adenovirus-mediated mda-7 gene expression in vivo. Oncogene. 2002;21:4558-4566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 39. | Nishikawa T, Ramesh R, Munshi A, Chada S, Meyn RE. Adenovirus-mediated mda-7 (IL24) gene therapy suppresses angiogenesis and sensitizes NSCLC xenograft tumors to radiation. Mol Ther. 2004;9:818-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Date K, Matsumoto K, Shimura H, Tanaka M, Nakamura T. HGF/NK4 is a specific antagonist for pleiotrophic actions of hepatocyte growth factor. FEBS Lett. 1997;420:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 166] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 41. | Wen J, Matsumoto K, Taniura N, Tomioka D, Nakamura T. Hepatic gene expression of NK4, an HGF-antagonist/angiogenesis inhibitor, suppresses liver metastasis and invasive growth of colon cancer in mice. Cancer Gene Ther. 2004;11:419-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 42. | Xin L, Xu R, Zhang Q, Li TP, Gan RB. Kringle 1 of human hepatocyte growth factor inhibits bovine aortic endothelial cell proliferation stimulated by basic fibroblast growth factor and causes cell apoptosis. Biochem Biophys Res Commun. 2000;277:186-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Xu R, Yao ZY, Xin L, Zhang Q, Li TP, Gan RB. NC1 domain of human type VIII collagen (alpha 1) inhibits bovine aortic endothelial cell proliferation and causes cell apoptosis. Biochem Biophys Res Commun. 2001;289:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Liu Y, Deisseroth A. Tumor vascular targeting therapy with viral vectors. Blood. 2006;107:3027-3033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Schiffelers RM, Ansari A, Xu J, Zhou Q, Tang Q, Storm G, Molema G, Lu PY, Scaria PV, Woodle MC. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 2004;32:e149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 652] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 46. | Chien PY, Wang J, Carbonaro D, Lei S, Miller B, Sheikh S, Ali SM, Ahmad MU, Ahmad I. Novel cationic cardiolipin analogue-based liposome for efficient DNA and small interfering RNA delivery in vitro and in vivo. Cancer Gene Ther. 2005;12:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 47. | Kondo M, Asai T, Katanasaka Y, Sadzuka Y, Tsukada H, Ogino K, Taki T, Baba K, Oku N. Anti-neovascular therapy by liposomal drug targeted to membrane type-1 matrix metalloproteinase. Int J Cancer. 2004;108:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Muramatsu T, Nakamura A, Park HM. In vivo electroporation: a powerful and convenient means of nonviral gene transfer to tissues of living animals (Review). Int J Mol Med. 1998;1:55-62. [PubMed] |
| 49. | Uesato M, Gunji Y, Tomonaga T, Miyazaki S, Shiratori T, Matsubara H, Kouzu T, Shimada H, Nomura F, Ochiai T. Synergistic antitumor effect of antiangiogenic factor genes on colon 26 produced by low-voltage electroporation. Cancer Gene Ther. 2004;11:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Liljeström P. Alphavirus expression systems. Curr Opin Biotechnol. 1994;5:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Mossman SP, Bex F, Berglund P, Arthos J, O'Neil SP, Riley D, Maul DH, Bruck C, Momin P, Burny A. Protection against lethal simian immunodeficiency virus SIVsmmPBj14 disease by a recombinant Semliki Forest virus gp160 vaccine and by a gp120 subunit vaccine. J Virol. 1996;70:1953-1960. [PubMed] |
| 52. | Zhang J, Asselin-Paturel C, Bex F, Bernard J, Chehimi J, Willems F, Caignard A, Berglund P, Liljeström P, Burny A. Cloning of human IL-12 p40 and p35 DNA into the Semliki Forest virus vector: expression of IL-12 in human tumor cells. Gene Ther. 1997;4:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Asselin-Paturel C, Lassau N, Guinebretière JM, Zhang J, Gay F, Bex F, Hallez S, Leclere J, Peronneau P, Mami-Chouaib F. Transfer of the murine interleukin-12 gene in vivo by a Semliki Forest virus vector induces B16 tumor regression through inhibition of tumor blood vessel formation monitored by Doppler ultrasonography. Gene Ther. 1999;6:606-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Chikkanna-Gowda CP, Sheahan BJ, Fleeton MN, Atkins GJ. Regression of mouse tumours and inhibition of metastases following administration of a Semliki Forest virus vector with enhanced expression of IL-12. Gene Ther. 2005;12:1253-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Logg CR, Tai CK, Logg A, Anderson WF, Kasahara N. A uniquely stable replication-competent retrovirus vector achieves efficient gene delivery in vitro and in solid tumors. Hum Gene Ther. 2001;12:921-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Sun Y, Finger C, Alvarez-Vallina L, Cichutek K, Buchholz CJ. Chronic gene delivery of interferon-inducible protein 10 through replication-competent retrovirus vectors suppresses tumor growth. Cancer Gene Ther. 2005;12:900-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Brody SL, Crystal RG. Adenovirus-mediated in vivo gene transfer. Ann N Y Acad Sci. 1994;716:90-101; discussion 101-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 124] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 58. | Xu ZL, Mizuguchi H, Sakurai F, Koizumi N, Hosono T, Kawabata K, Watanabe Y, Yamaguchi T, Hayakawa T. Approaches to improving the kinetics of adenovirus-delivered genes and gene products. Adv Drug Deliv Rev. 2005;57:781-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Wolf JK, Bodurka DC, Gano JB, Deavers M, Ramondetta L, Ramirez PT, Levenback C, Gershenson DM. A phase I study of Adp53 (INGN 201; ADVEXIN) for patients with platinum- and paclitaxel-resistant epithelial ovarian cancer. Gynecol Oncol. 2004;94:442-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Han DM, Huang ZG, Zhang W, Yu ZK, Wang Q, Ni X, Chen XH, Pan JH, Wang H. [Effectiveness of recombinant adenovirus p53 injection on laryngeal cancer: phase I clinical trial and follow up]. Zhonghua Yixue Zazhi. 2003;83:2029-2032. [PubMed] |
| 61. | Zhang SW, Xiao SW, Liu CQ, Sun Y, Su X, Li DM, Xu G, Cai Y, Zhu GY, Xu B. [Treatment of head and neck squamous cell carcinoma by recombinant adenovirus-p53 combined with radiotherapy: a phase II clinical trial of 42 cases]. Zhonghua Yixue Zazhi. 2003;83:2023-2028. [PubMed] |
| 62. | Chen CB, Pan JJ, Xu LY. [Recombinant adenovirus p53 agent injection combined with radiotherapy in treatment of nasopharyngeal carcinoma: a phase II clinical trial]. Zhonghua Yixue Zazhi. 2003;83:2033-2035. [PubMed] |
| 63. | Mathis JM, Stoff-Khalili MA, Curiel DT. Oncolytic adenoviruses - selective retargeting to tumor cells. Oncogene. 2005;24:7775-7791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 64. | Alba R, Bosch A, Chillon M. Gutless adenovirus: last-generation adenovirus for gene therapy. Gene Ther. 2005;12 Suppl 1:S18-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 180] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 65. | Smith-Arica JR, Bartlett JS. Gene therapy: recombinant adeno-associated virus vectors. Curr Cardiol Rep. 2001;3:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 66. | Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14:316-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 654] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 67. | Isayeva T, Ren C, Ponnazhagan S. Recombinant adeno-associated virus 2-mediated antiangiogenic prevention in a mouse model of intraperitoneal ovarian cancer. Clin Cancer Res. 2005;11:1342-1347. [PubMed] |
| 68. | Yanamandra N, Kondraganti S, Gondi CS, Gujrati M, Olivero WC, Dinh DH, Rao JS. Recombinant adeno-associated virus (rAAV) expressing TFPI-2 inhibits invasion, angiogenesis and tumor growth in a human glioblastoma cell line. Int J Cancer. 2005;115:998-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 69. | Eder JP Jr, Supko JG, Clark JW, Puchalski TA, Garcia-Carbonero R, Ryan DP, Shulman LN, Proper J, Kirvan M, Rattner B. Phase I clinical trial of recombinant human endostatin administered as a short intravenous infusion repeated daily. J Clin Oncol. 2002;20:3772-3784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 177] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 70. | Herbst RS, Hess KR, Tran HT, Tseng JE, Mullani NA, Charnsangavej C, Madden T, Davis DW, McConkey DJ, O'Reilly MS. Phase I study of recombinant human endostatin in patients with advanced solid tumors. J Clin Oncol. 2002;20:3792-3803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 203] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 71. | Herbst RS, Mullani NA, Davis DW, Hess KR, McConkey DJ, Charnsangavej C, O'Reilly MS, Kim HW, Baker C, Roach J. Development of biologic markers of response and assessment of antiangiogenic activity in a clinical trial of human recombinant endostatin. J Clin Oncol. 2002;20:3804-3814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 120] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 72. | Thomas JP, Arzoomanian RZ, Alberti D, Marnocha R, Lee F, Friedl A, Tutsch K, Dresen A, Geiger P, Pluda J. Phase I pharmacokinetic and pharmacodynamic study of recombinant human endostatin in patients with advanced solid tumors. J Clin Oncol. 2003;21:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 174] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 73. | Clamp AR, Jayson GC. The clinical potential of antiangiogenic fragments of extracellular matrix proteins. Br J Cancer. 2005;93:967-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 74. | Abdollahi A, Hlatky L, Huber PE. Endostatin: the logic of antiangiogenic therapy. Drug Resist Updat. 2005;8:59-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 75. | Tjin Tham Sjin RM, Naspinski J, Birsner AE, Li C, Chan R, Lo KM, Gillies S, Zurakowski D, Folkman J, Samulski J. Endostatin therapy reveals a U-shaped curve for antitumor activity. Cancer Gene Ther. 2006;13:619-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 76. | Sangro B, Mazzolini G, Ruiz J, Herraiz M, Quiroga J, Herrero I, Benito A, Larrache J, Pueyo J, Subtil JC. Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors. J Clin Oncol. 2004;22:1389-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 233] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 77. | Sangro B, Melero I, Qian C, Prieto J. Gene therapy of cancer based on interleukin 12. Curr Gene Ther. 2005;5:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 78. | Scappaticci FA. Mechanisms and future directions for angiogenesis-based cancer therapies. J Clin Oncol. 2002;20:3906-3927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 156] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 79. | Abdollahi A, Lipson KE, Sckell A, Zieher H, Klenke F, Poerschke D, Roth A, Han X, Krix M, Bischof M. Combined therapy with direct and indirect angiogenesis inhibition results in enhanced antiangiogenic and antitumor effects. Cancer Res. 2003;63:8890-8898. [PubMed] |
| 80. | Huber PE, Bischof M, Jenne J, Heiland S, Peschke P, Saffrich R, Gröne HJ, Debus J, Lipson KE, Abdollahi A. Trimodal cancer treatment: beneficial effects of combined antiangiogenesis, radiation, and chemotherapy. Cancer Res. 2005;65:3643-3655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 81. | Jin F, Xie Z, Kuo CJ, Chung LW, Hsieh CL. Cotargeting tumor and tumor endothelium effectively inhibits the growth of human prostate cancer in adenovirus-mediated antiangiogenesis and oncolysis combination therapy. Cancer Gene Ther. 2005;12:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |