Published online Nov 14, 2006. doi: 10.3748/wjg.v12.i42.6828
Revised: September 15, 2006
Accepted: September 26, 2006
Published online: November 14, 2006
AIM: To verify the impairment of the hepatic lipid metabolism in prehepatic portal hypertension.
METHODS: The concentrations of free fatty acids, diacylglycerol, triglycerides, and phospholipids were assayed by using D-[U-14C] glucose incorporation in the different lipid fractions and thin-layer chromatography and cholesterol was measured by spectrophotometry, in liver samples of Wistar rats with partial portal vein ligation at short- (1 mo) and long-term (1 year) (i.e. portal hypertensive rats) and the control rats.
RESULTS: In the portal hypertensive rats, liver phospholipid synthesis significantly decreased (7.42 ± 0.50 vs 4.70 ± 0.44 nCi/g protein; P < 0.01) and was associated with an increased synthesis of free fatty acids (2.08 ± 0.14 vs 3.36 ± 0.33 nCi/g protein; P < 0.05), diacylglycerol (1.93 ± 0.2 vs 2.26 ± 0.28 nCi/g protein), triglycerides (2.40 ± 0.30 vs 4.49 ± 0.15 nCi/g protein) and cholesterol (24.28 ± 2.12 vs 57.66 ± 3.26 mg/g protein; P < 0.01).
CONCLUSION: Prehepatic portal hypertension in rats impairs the liver lipid metabolism. This impairment consists in an increase in lipid deposits (triglycerides, diacylglycerol and cholesterol) in the liver, accompanied by a decrease in phospholipid synthesis.
- Citation: Aller MA, Vara E, García C, Nava MP, Angulo A, Sánchez-Patán F, Calderón A, Vergara P, Arias J. Hepatic lipid metabolism changes in short- and long-term prehepatic portal hypertensive rats. World J Gastroenterol 2006; 12(42): 6828-6834
- URL: https://www.wjgnet.com/1007-9327/full/v12/i42/6828.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i42.6828
The most used experimental model to study prehepatic portal hypertension is that obtained by calibrated stenosis of the portal vein in the rat[1,2]. It has been suggested that this experimental model has a homogeneous evolution, with a narrow range of portal hypertension, degree of porto-systemic shunts and hepatic atrophy[2-4]. However, prehepatic portal hypertensive rats have a far from uniform evolution, since they can present wide variability in both the type and degree of portosystemic collateral circulation which is developed[5] as well as in the degree of liver atrophy[5,6].
The different evolution of hepatic weight in rats with prehepatic portal hypertension is an interesting finding, since it demonstrates the existence of a heterogeneous hepatic response in this experimental model. A previous histological study of the liver was performed in order to verify whether the existence of a liver disease could justify this wide spectrum of liver weight. This study demonstrated that prehepatic portal hypertension in the rat produces fatty infiltration of the liver. Moreover, this fat accumulation in hepatocytes progresses from a short-term (1 mo) to a long-term (1 year) evolutive stage of portal hypertension[7].
In order to study hepatic lipid metabolism in these evolutive stages of experimental prehepatic portal hypertension, we determined the concentrations of free fatty acids (FFA), diacylglycerides (DG), triglycerides (TG), phospholipids (PL) and cholesterol (Ch) in the liver of the rats with calibrated stenosis of the portal vein, both after short-term (1 mo) and long-term (1 year) evolution.
Male Wistar rats, weighing 250-300 g, were obtained from the Vivarium of the Complutense University of Madrid. The animals were anesthetized by im injection of ketamine (100 mg/kg) and xylacine (12 mg/kg). The animals were fed standard laboratory rodents’ diet (rat/mouse AO4 maintenance diet: 17.6% proteins, 43.3% starch 2.5% lipids, Panlab, Spain) and water ad libitum. They were housed in a temperature- (22 ± 2°C), humidity- (65%-70%) and light-controlled room (light, 7:30 am-7:30 pm) in groups of three to four animals.
The experimental procedures employed in this study were in accordance with the principles and practices of the 1986 European Guide for the Care and Use of Laboratory Animals, published in Spain in Royal Decree 1201/2005.
The animals were randomly divided into four groups: groupI(n = 5) consisted of control rats at 1 mo of evolution; groupII(n = 11) consisted of triple calibrated portal vein stenosis (TPVS) rats at 1 mo of postoperative evolution; group III (n = 4) consisted of control rats at 1 year of evolution; and group IV (n = 6) consisted of TPVS rats at 1 year of postoperative evolution. The control rats (groupIand III) did not undergo any operative intervention. All the animals were sacrificed by exsanguination and body (BW), liver (LW) and spleen (SW) weights were determined.
The surgical procedure used to establish portal hypertension by triple partial ligation of the portal vein (TPVS) has been described previously[8]. In brief, the portal vein was isolated and three partial ligatures were performed in its superior, medial and inferior portions. The stenoses were calibrated by a simultaneous ligature (4-0 silk) around the portal vein with a 20-gauge needle. The midline abdominal incision was closed in two layers with catgut and 3-0 silk.
Portosystemic collateral circulation was studied as follows: first, a midline abdominal incision with a large bilateral subcostal extension was performed and then the areas in which the collateral venous circulation was developed (i.e. the splenorenal, gastroesophageal, colorectal and hepatic hilum) were carefully studied for the presence of increased collateral veins[9].
Splenic pulp pressure, an indirect measurement of portal pressure (PP), was measured by inserting a fluid-filled 20-gauge needle into the splenic parenchyma[10]. The needle was joined to a PE-50 tube and then connected to a pressure recorder (PowerLab 200 ML 201) and to a transducer (Sensonor SN-844) with a Chart V 4.0 computer program (ADI Instruments) and was calibrated before each experiment. The pressure reading was considered satisfactory when a stable recording was produced and respiratory variations were observed. Previous studies have demonstrated the excellent correlation between splenic pulp pressure and PP[11].
Chemicals: D-[U-14C] glucose was purchased from the Radiochemical Centre (Amersham, Buckinghamshire, UK). Standard lipids and 2’, 7’-dichlorofluorescein were purchased from Sigma Chemical Co. (Saint Louis, MO).
Lipid synthesis: “De novo” lipid synthesis was measured by the incorporation of either D-[U-14C] glucose (12.5 Ci/mol) into TG, PL, DG and FFA. Labeled glucose was injected after dissolving in saline (5 mL/kg). All the rats were sacrificed 24 h after treatment, and the livers were excised and washed. After specific extraction, lipids were determined. Briefly, 50 mg of tissue was rapidly frozen in acetone chilled with dry ice. After addition of 0.7 mL of acid methanol (methanol/HCl, 100:1) to the tubes, the content was homogenized, centrifuged and sonicated in an MSE ultrasonic disintegrator (Branson, Danburry, CT). The lipids were then extracted with 1.3 mL of chloroform and 0.4 mL of salt solution[12] for 1 h at room temperature. The organic phase was then washed three times with 1.0 mL of the aqueous phase of a system composed of chloroform/methanol/salt solution/concentrated HCL (266:133:100:1). The organic phase was dried under N2 and re-dissolved in 40 μL of chloroform/methanol (2:1). A sample (30 μL) was then applied to precoated plates of silica gel 60 (20 cm × 20 cm; Merck) previously activated for 1 h at 110°C. Lipid separation was performed by unidimensional chromatography. Then, the plates were sprayed with 2’ 7’-dichlorofluorescein, and the following spots were detected under UV light and identified with suitable markers: PL, TG, DG and FFA. Each spot was scraped off into a scintillation vial, and its radioactivity was measured.
Cholesterol determination was performed with a specific commercial kit (Spinreact, Richmond, 1972)[13] based on the action of the cholesterol enzyme stearase which hydrolyzed the esters present in the sample, giving free cholesterol and fatty acids. A subsequent enzymatic oxidation using the cholesterol enzyme oxidase formed hydrogen peroxide and cholesterine. The peroxide was evaluated by the Trinder reaction by a chromogene, in the presence of peroxidase, forming a quinonimine with a red coloring. The intensity of this color was proportional to the cholesterol concentration in the sample. Finally, colorimetric determination was performed in a spectrophotometer (Eppendorf, model Biophotometer) at a wavelength of 500 nm.
This was done by the colorimetric method described by Bradford[14]. This method is based on the binding of Coomassie Blue to proteins. This binding causes a displacement of the peak absorption of the dye from 465 to 595 nm. Absorbance is measured in the samples at the latter wavelength against a known reference curve. The protein complex dye has a high coefficient of extinction, which gives it a high sensitivity at measuring the protein.
Protein determination was carried out because the concentration of lipids and cholesterol is expressed in relation to the concentration of liver proteins.
Results were expressed as means ± SD. ANOVA and Duncan tests were used for the statistical comparison of the variables between the four groups. The results were considered statistically significant when P < 0.05.
The animals with portal hypertension (groupsIIand IV) showed a markedly less body weight increase than that found in the control animals (groupsIand III): 82.49 ± 29.08 g vs 64.72 ± 29.32 g and 268.02 ± 78.03 g vs 361.02 ± 58.32 g, P < 0.05). The animals with TPVS showed a decrease in liver weight (9.98 ± 1.55 g in group II vs 12.01 ± 2.64 g in groupI, P < 0.05; 15.84 ± 2.02 g in group IV vs 16.77 ± 2.87 g in group III).
All animals with TPVS showed splenomegaly: 1.00 ± 0.16 g in group II vs 0.49 ± 0.06 g in groupI(P < 0.05) and 1.43 ± 0.84 in group IV vs 0.91 ± 0.10 g in group III (P < 0.05) (Table 1).
All the TPVS rats showed characteristic features of splanchnic venous congestion with dilation of mesenteric venous circulation. The portal pressure in groupsIIand IV was obviously higher compared to groupsIand III (14.63 ± 2.84 mmHg vs 7.32 ± 1.15 mmHg; and 9.05 ± 2.84 mmHg vs 6.72 ± 1.52 mmHg, respectively; P < 0.05) (Table 1).
In the rats with TPVS (groupsIIand IV), splenorenal portosystemic collateral circulation was the commonest type (groupII: n = 11, 100%; group IV: n = 4, 66%).
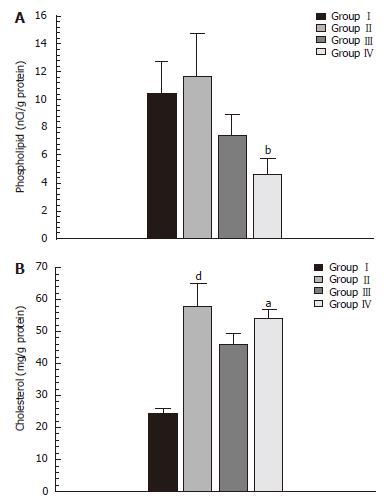
The hepatic concentration of phospholipids underwent a significant increase (P < 0.005) in the TPVS animals after one year (group IV) compared to the control group (group III) (Figure 1).
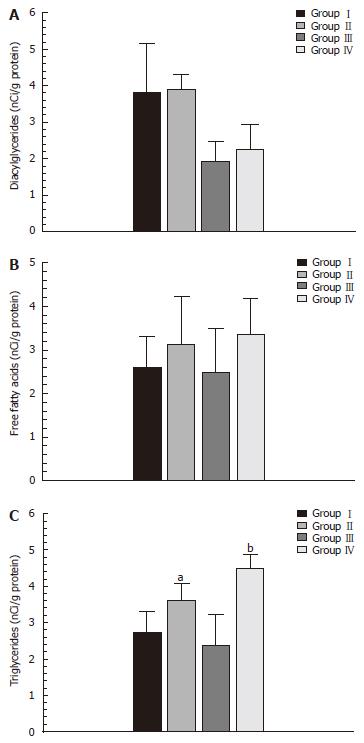
The increase in diacylglyceride in the both groupsII(TPVS 1 evolutive month) and IV (TPVS 1 evolutive year) was not statistically significant compared to their corresponding controls. Moreover, although the hepatic concentrations of FFA increased in the animal series with portal hypertension (groupsIIand IV), this increase was not significant compared to their corresponding controls (Figure 2).
A statistically significant increase in hepatic concentra-tions of TG (Figure 2) and Ch (Figure 1) in the animals with portal hypertension (groupsIIand IV) compared to their corresponding control groups (groupIand III, respectively) was observed.
Our study demonstrated that prehepatic portal hyperten-sion in the rats, for both in the short-term (1 mo) and long-term (1 year) period, produced hepatic accumulation of triglycerides and cholesterol. Our results provided further evidence to support a long-term evolution (1 year) in the production of a severe drop in hepatic phospholipids (Figures 1 and 2).
Liver steatosis could be the cause of the hepatomegaly described in the evolution of prehepatic hypertension in the rat[5,6]. If so, portal stenosis ligation could be used to make a model of steatosis as well as to obtain an experimental model of portal hypertension[7].
Hepatic steatosis alone is thought to be the most common form of non-alcoholic fatty liver disease (NAFLD) and is considered to be “benign”, but not quiescent[15]. The NAFLD spectrum is wide and ranges from simple fat accumulation in hepatocytes (fatty liver), without biochemical or histological evidence of inflammation or fibrosis, to fat accumulation plus necro-inflammatory activity with or without fibrosis (non-alcoholic steatohepatitis, NASH) and the development of advanced liver fibrosis or cirrhosis (cirrhotic stage)[15-19].
However, although a progressive hepatocytic fatty infiltration occurs during chronic evolution in TPVS rats, this is not associated with histological signs of inflammation or fibrosis. Therefore, hepatic steatosis could be considered as a “benign” type of the broad spectrum of NAFLD occurring in these rats with prehepatic portal hypertension[7].
The lipid content of hepatocytes is regulated by the integrated activities of cellular enzymes that catalyze lipid uptake, synthesis, oxidation and export. Fat accumulates within the hepatocytes when the “input” (either uptake or synthesis) of fatty acids to hepatocytes exceeds their “output” (oxidation and export)[20].
Different factors, extrahepatic and intrahepatic, can impair both regulator mechanisms and, therefore, promote triglyceride and cholesterol accumulation in the liver of rats with short-term (1 mo) and long-term (1 year) prehepatic portal hypertension. These factors could be divided into neuroendocrine and immunologic for their etiopathogenic study. The possible neuroendocrine factors involved in fat accumulation within the hepatocytes include an increase in the components of the neuroendocrine response to TPVS stress: plasma corticoids[8], catecholamines[21,22] and glucagon[23] levels in portal hypertensive rats. In the neuroendocrine response to stress, a promotion of lipolysis in adipose tissues is also produced with increased plasma free fatty acid concentrations and subsequently, an excess “input” of fatty acids to the liver in short-term prehepatic portal hypertensive rats. In turn, the expanded pool of NAFLD would induce accumulation of triglycerides within the cytoplasm of hepatocytes as fat microvesicles[24-26]. Insulin resistance causes an insufficient suppression of hormone-sensitive lipase activity and reduced glucose uptake in adipose tissue. This results in enhanced lipolysis and increased fatty acid flux into the plasma non-sterified fatty acid pool[26]. It has been demonstrated that approximately 60% of triacylglicerides accumulated in the liver are derived from the plasma non-sterified fatty acids pool. The enhanced adipocyte lipolysis, in turn, is associated with an increased activity of lipogenic enzymes, contributing to hepatic steatosis[26]. Insulin resistance induced by glucocorticoids could also contribute to hepatic steatosis by favouring peripheral lipolysis and hepatic uptake of fatty acids[25,27].
Therefore, in the presence of insulin resistance, increased lipolysis results in the continuous release of long-chain fatty acids (LCFA) from adipose tissue, leading to a characteristic increase in the circulating LCFA concentration[26,28]. When this process occurs in fat-swollen omental and mesenteric fat cells, as it occurs in portal hypertension in the rat, this results in an especially large increase in the mass of LCFAs entering the portal circulation and being delivered directly to the liver[28]. The increase of FFA delivery to the liver, in turn, causes hepatic insulin resistance, as well as tumor necrosis factor alpha (TNF-α) production[29]. When TNF-α is overproduced relative to adiponectin, adiponectin activity declines. Reduced adiponectin activity also favors hepatic insulin resistance and fat accumulation[29,30]. In addition, low adiponectin activity permits TNF-α activity, which induces insulin resistance[29,30].
The cytokine TNF-α is among the most important immunologic factors potentially involved in the production of hepatic steatosis[17,31,32]. The increase in TNF-α activity, which increases nitric oxide (NO) and carbon monoxide (CO) levels, two potent vasodilators, is at least partially responsible for development of the hyperdynamic systemic and splanchnic state that contributes to maintaining an elevated portal pressure in early evolutive periods of experimental prehepatic portal hypertension[33-37]. However, TNF-α and TNF-regulated cytokines are also considered as effector molecules in liver damage in the animals and patients with non-alcoholic liver diseases, ranging from steatosis to cirrhosis[17,32]. It has been accepted that liver injury requires at least two “hits”: One that increases exposure of the hepatocytes to TNF-α and another one that interferes with the normal ability of fat, but renders the liver more vulnerable to a second insult because the hepatocytes became sensitized to the TNF-mediated cell death[32,38].
Although Kupffer cells are a major source of liver-derived pro-inflammatory cytokines, other liver cells can also synthesize cytokines. Particularly, fat accumulation within hepatocytes triggers intracellular signals, activates nuclear factor kappa B (NF-κB) and induces excessive hepatocyte production and insulin resistance[39].
On the other hand, TNF-α has been proposed as causing the hyperlipidemia that occurs in different pathological situations. It has also been shown that TNF-α decreases the level of lipase activity against lipoproteins in adipose tissue[40]. In addition, TNF-α-induced increase in lipid synthesis is accompanied by both an increase in the activity of acetyl coenzyme A carboxylase (a rate-limiting enzyme for fatty acid synthesis) and a rise in its allosteric activator, citrate. This suggests that a relative increase in the activity of this key regulatory enzyme may be one of the mechanisms responsible for. There is also evidence that the rate of triacylglycerol synthesis is controlled by regular coordination of the activities of phosphatidate phosphatase and diacylglycerol acyl transferase[41] .The activities of both enzymes are increased in many conditions of stress, including metabolic stress and exposure to ethanol and glucocorticoids[41].
Accumulated evidence suggests a major role for mitochondrial dysfunction in steatosis[24]. Also, oxidative stress with formation of reactive oxygen species (ROS) increases in fatty livers[42,43]. This abundant ROS formation may oxidize the unsaturated lipids of fat deposits to cause lipid peroxidation[34]. Both lipid peroxidation products and TNF-α may damage mitochondria and directly attack and inactivate respiratory chain components[15,24,25,44,45]. ROS and lipid peroxidation can also lead to ultrastructural mitochondrial alterations and megamitochondria[24,46] and hepatocyte necrosis or apoptosis[44]. The pro-inflammatory cytokines, particularly TNF-α, induce iNOS hyperactivity with an increased NO synthesis. ROS, on binding to NO, form peroxynitrite and this reactive nitrogen radical could bind proteins of the mitochondrial respiratory chain, resulting in its functional impairment with a decrease in oxidative phosphorylation and, consequently, of ATP[47].
ROS, in turn, can cause NF-κB activation, which induces the synthesis of TNF-α, and this cytokine damages mitochondria and increases mitochondrial ROS formation[24]. Mitochondrial dysfunction can, therefore, reduce oxidative phosphorylation and the ATP production that occurs in hepatic steatosis[25,46] and would explain the low resistance of steatotic livers to a second aggressive stimulus or “second hit”[17].
Since the accumulation of triglycerides and cholesterol in the hepatocytes persisted in the long-term (1 year) evolutive stage of prehepatic portal hypertension, possibly, the etiopathogenic mechanisms involved in its production could also persist. Moreover, in this evolutive stage of experimental prehepatic portal hypertension, the increase in lipid deposits associated with a decline in the synthesis of lipid complexes would be associated with the decreased synthesis of lipid complexes, such as phospholipids (PL) (Figure 1), which would worsen the sensitivity to liver injury. Phospholipids are a critical part of the structure of eukaryotic cell membranes. When PL synthesis was examined, we found a significant decrease after one year. Since PC is the main PL in liver membranes, the observed decrease in PL could reflect a decrease in PC synthesis. PC synthesis in the liver mainly occurs through two pathways, the cytidine diphosphate (CDP) choline pathway and the methylation of phosphat-idylethanolamine. In the CDP-choline pathway, the most important pathway, cytidine diphosphate phosphocholine cytidyl transferase catalyses the rate-limiting step. A possible mechanism for the induced PL decrease could be through the release of cytidyl transferase from the membranes, probably mediated by the release of inflammatory mediators, including glucocorticoids and/or cytokines[47].
The excess liver storage of TG and cholesterol results in steatosis and also provides substrates for lipid peroxidation[48]. The accumulation of cholesterol and the PL decrease have been associated with the metabolic syndrome[49]. This classical lipid changes may be envisioned as a highly conserved evolutionary response aimed at tissue repair[50].
An inflammatory hypothesis has been proposed in relation to the etiopathogeny of the portal hypertensive enteropathy. This consists in the involvement of the mast cells[51,52] and the inflammatory mediators released by them, of which histamine would be one of the most important. Histamine, as an effective metabolite, could affect the lipid metabolism in liver cells by facilitating the liver cells’ accumulation of lipid droplets (fatty liver)[53]. Therefore, in this experimental model of prehepatic portal hypertension, histamine released by activated mast cells could also be involved in producing a fatty liver.
In summary, prehepatic portal hypertension in rats produces changes in liver lipid metabolism, which are similar to those associated with chronic inflammatory conditions and with sepsis[54].
We thank Pedro Cuesta from the Centro de Cálculo of Complutense University of Madrid for the statistical study and Caroline Coope for English translation of the manuscript.
S- Editor Wang GP L- Editor Kumar M E- Editor Bai SH
| 1. | Chojkier M, Groszmann RJ. Measurement of portal-systemic shunting in the rat by using gamma-labeled microspheres. Am J Physiol. 1981;240:G371-G375. [PubMed] |
| 2. | Bosch J, Pizcueta P, Feu F, Fernández M, García-Pagán JC. Pathophysiology of portal hypertension. Gastroenterol Clin North Am. 1992;21:1-14. [PubMed] |
| 3. | MacMathuna P, Vlavianos P, Westaby D, Williams R. Pathophysiology of portal hypertension. Dig Dis. 1992;10 Suppl 1:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Lin HC, Yang MC, Hou MC, Li SM, Huang YT, Yu PC, Tsai YT, Lee SD. Effects of long-term administration of octreotide in portal vein-stenosed rats. Hepatology. 1996;23:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Aller Reyero MA, Diéguez Fernández B, Nava Hidalgo MP, Cuesta Alvaro P, Sánchez García M, Duran Giménez-Rico HJ, Llamas Matías MA, Arias Pérez J. [Development of pre-hepatic portal hypertension of the rat]. An Med Interna. 2002;19:341-351. [PubMed] |
| 6. | Van Thiel DH, Gavaler JS, Cobb CF, McClain CJ. An evaluation of the respective roles of portosystemic shunting and portal hypertension in rats upon the production of gonadal dysfunction in cirrhosis. Gastroenterology. 1983;85:154-159. [PubMed] |
| 7. | Alonso MJ, Aller MA, Corcuera MT, Nava MP, Gömez F, Angulo A, Arias J. Progressive hepatocytic fatty infiltration in rats with prehepatic portal hypertension. Hepatogastroenterology. 2005;52:541-546. [PubMed] |
| 8. | Monterde G, Rodríguez-Fabian G, Vara E, López L, Arias J, Aller MA, Arias J. Increased plasma levels of corticosterone and prolactin and decreased T3 and T4 levels in short-term prehepatic portal hypertension in rats. Dig Dis Sci. 2000;45:1865-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Diéguez B, Aller MA, Nava MP, Palma MD, Arias JL, López L, Arias J. Chronic portal hypertension in the rat by triple-portal stenosing ligation. J Invest Surg. 2002;15:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Castañeda B, Debernardi-Venon W, Bandi JC, Andreu V, Pérez-del-Pulgar S, Moitinho E, Pizcueta P, Bosch J. The role of portal pressure in the severity of bleeding in portal hypertensive rats. Hepatology. 2000;31:581-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Kravetz D, Sikuler E, Groszmann RJ. Splanchnic and systemic hemodynamics in portal hypertensive rats during hemorrhage and blood volume restitution. Gastroenterology. 1986;90:1232-1240. [PubMed] |
| 12. | Tamarit-Rodríguez J, Vara E, Tamarit J. Starvation-induced changes of palmitate metabolism and insulin secretion in isolated rat islets stimulated by glucose. Biochem J. 1984;221:317-324. [PubMed] |
| 13. | Richmond W. Proceedings: The development of an enzymic technique for the assay of cholesterol in biological fluids. Clin Sci Mol Med. 1974;46:6P-7P. [PubMed] |
| 14. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189576] [Cited by in RCA: 157401] [Article Influence: 3212.3] [Reference Citation Analysis (0)] |
| 15. | McCullough AJ. Update on nonalcoholic fatty liver disease. J Clin Gastroenterol. 2002;34:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 218] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Brunt EM. Alcoholic and nonalcoholic steatohepatitis. Clin Liver Dis. 2002;6:399-420, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Solga SF, Diehl AM. Non-alcoholic fatty liver disease: lumen-liver interactions and possible role for probiotics. J Hepatol. 2003;38:681-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Cortez-Pinto H, Camilo ME. Non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH): diagnosis and clinical course. Best Pract Res Clin Gastroenterol. 2004;18:1089-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Jansen PL. Non-alcoholic steatohepatitis. Eur J Gastroenterol Hepatol. 2004;16:1079-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Koteish A, Diehl AM. Animal models of steatosis. Semin Liver Dis. 2001;21:89-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 358] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 21. | Kiel JW, Pitts V, Benoit JN, Granger DN, Shepherd AP. Reduced vascular sensitivity to norepinephrine in portal-hypertensive rats. Am J Physiol. 1985;248:G192-G195. [PubMed] |
| 22. | Bomzon A, Blendis LM. Vascular reactivity in experimental portal hypertension. Am J Physiol. 1987;252:G158-G162. [PubMed] |
| 23. | Sikuler E, Groszmann RJ. Hemodynamic studies in long- and short-term portal hypertensive rats: the relation to systemic glucagon levels. Hepatology. 1986;6:414-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Fromenty B, Robin MA, Igoudjil A, Mansouri A, Pessayre D. The ins and outs of mitochondrial dysfunction in NASH. Diabetes Metab. 2004;30:121-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 190] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 25. | Pessayre D, Fromenty B, Mansouri A. Mitochondrial injury in steatohepatitis. Eur J Gastroenterol Hepatol. 2004;16:1095-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Tamura S, Shimomura I. Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1139-1142. [PubMed] |
| 27. | Choudhury J, Sanyal AJ. Insulin resistance in NASH. Front Biosci. 2005;10:1520-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Bradbury MW, Berk PD. Lipid metabolism in hepatic steatosis. Clin Liver Dis. 2004;8:639-671, xi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Diehl AM. Lessons from animal models of NASH. Hepatol Res. 2005;33:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3510] [Cited by in RCA: 3503] [Article Influence: 146.0] [Reference Citation Analysis (0)] |
| 31. | Vara E, Arias-Díaz J, Torres-Melero J, García C, Rodríguez JM, Balibrea JL. Effect of different sepsis-related cytokines on lipid synthesis by isolated hepatocytes. Hepatology. 1994;20:924-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 685] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 33. | Lopez-Talavera JC, Merrill WW, Groszmann RJ. Tumor necrosis factor alpha: a major contributor to the hyperdynamic circulation in prehepatic portal-hypertensive rats. Gastroenterology. 1995;108:761-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 121] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Lopez-Talavera JC, Cadelina G, Olchowski J, Merrill W, Groszmann RJ. Thalidomide inhibits tumor necrosis factor alpha, decreases nitric oxide synthesis, and ameliorates the hyperdynamic circulatory syndrome in portal-hypertensive rats. Hepatology. 1996;23:1616-1621. [PubMed] |
| 35. | Wiest R, Groszmann RJ. Nitric oxide and portal hypertension: its role in the regulation of intrahepatic and splanchnic vascular resistance. Semin Liver Dis. 1999;19:411-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Palma MD, Aller MA, Vara E, Nava MP, Garcia C, Arias-Diaz J, Balibrea JL, Arias J. Portal hypertension produces an evolutive hepato-intestinal pro- and anti-inflammatory response in the rat. Cytokine. 2005;31:213-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Aller MA, Vara E, Garcia C, Palma MD, Arias JL, Nava MP, Arias J. Proinflammatory liver and antiinflammatory intestinal mediators involved in portal hypertensive rats. Mediators Inflamm. 2005;2005:101-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Diehl AM. Cytokine regulation of liver injury and repair. Immunol Rev. 2000;174:160-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 233] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 39. | Rao MS, Reddy JK. Peroxisomal beta-oxidation and steatohepatitis. Semin Liver Dis. 2001;21:43-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 141] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 40. | Kawakami M, Pekala PH, Lane MD, Cerami A. Lipoprotein lipase suppression in 3T3-L1 cells by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci USA. 1982;79:912-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 165] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Tijburg LB, Geelen MJ, van Golde LM. Regulation of the biosynthesis of triacylglycerol, phosphatidylcholine and phosphatidylethanolamine in the liver. Biochim Biophys Acta. 1989;1004:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 183] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 42. | Uzun H, Simsek G, Aydin S, Unal E, Karter Y, Yelmen NK, Vehid S, Curgunlu A, Kaya S. Potential effects of L-NAME on alcohol-induced oxidative stress. World J Gastroenterol. 2005;11:600-604. [PubMed] |
| 43. | de Oliveira CP, Simplicio FI, de Lima VM, Yuahasi K, Lopasso FP, Alves VA, Abdalla DS, Carrilho FJ, Laurindo FR, de Oliveira MG. Oral administration of S-nitroso-N-acetylcysteine prevents the onset of non alcoholic fatty liver disease in rats. World J Gastroenterol. 2006;12:1905-1911. [PubMed] |
| 44. | Boczkowski J, Lisdero CL, Lanone S, Carreras MC, Aubier M, Poderoso JJ. Peroxynitrite-mediated mitochondrial dysfunction. Biol Signals Recept. 2001;10:66-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Cortez-Pinto H, de Moura MC, Day CP. Non-alcoholic steatohepatitis: from cell biology to clinical practice. J Hepatol. 2006;44:197-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 46. | Prieto I, Jiménez F, Aller MA, Nava MP, Vara E, Garcia C, Arias J. Tumor necrosis factor-alpha, interleukin-1beta and nitric oxide: induction of liver megamitochondria in prehepatic portal hypertensive rats. World J Surg. 2005;29:903-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Caldwell SH, Swerdlow RH, Khan EM, Iezzoni JC, Hespenheide EE, Parks JK, Parker WD Jr. Mitochondrial abnormalities in non-alcoholic steatohepatitis. J Hepatol. 1999;31:430-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 351] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 48. | Pelech SL, Vance DE. Regulation of phosphatidylcholine biosynthesis. Biochim Biophys Acta. 1984;779:217-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 311] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 49. | Bradbury MW. Lipid metabolism and liver inflammation. I. Hepatic fatty acid uptake: possible role in steatosis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G194-G198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 256] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 50. | Esteve E, Ricart W, Fernández-Real JM. Dyslipidemia and inflammation: an evolutionary conserved mechanism. Clin Nutr. 2005;24:16-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 328] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 51. | Diez-Arias JA, Aller MA, Palma MD, Arias JL, Muñiz E, Sánchez M, Arias J. Increased duodenal mucosa infiltration by mast cells in rats with portal hypertension. Dig Surg. 2001;18:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Prieto I, Aller MA, Santamaría L, Nava MP, Madero R, Pérez-Robledo JP, Arias J. Prehepatic portal hypertension produces increased mast cell density in the small bowel and in mesenteric lymph nodes in the rat. J Gastroenterol Hepatol. 2005;20:1025-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Sasaguri Y, Tanimoto A. Role of macrophage-derived histamine in atherosclerosis-- chronic participation in the inflammatory response --. J Atheroscler Thromb. 2004;11:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Wu A, Hinds CJ, Thiemermann C. High-density lipoproteins in sepsis and septic shock: metabolism, actions, and therapeutic applications. Shock. 2004;21:210-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 140] [Article Influence: 6.7] [Reference Citation Analysis (0)] |