INTRODUCTION
CAPE (2-phenylethyl 3(3,4-dihdroxyphenyl)-2-propenoate), exhibits a broad spectrum of biological activities antibacterial[1], anti-inflammatory[2], antiviral[3], antiatherosclerotic[4], antiproliferative[5], neuroprotective[6-8] and antitumoral actions[9]. In in vivo models, CAPE has been identified as an experimental antineoplastic agent by inhibition of colon Aberrant Crypt Foci formation[10], mice skin tumor induction[11,12], intestinal tumor in Min/+ mice[13]; and, recently, we demonstrated that CAPE inhibits induction of AHF at a promotion stage of a chemical hepatocarcinogenesis model in rats[14].
The mechanism by which CAPE exerts its anti-carcinogenic effect is not completely known. In our previous work of the inhibition of promotion by this substance, we hypothesized that the activation of NF-kappaB induced by reactive oxygen species (ROS) generation produced by the metabolism of 2-acetamidofluorene (2-AAF) is inhibited by CAPE through its antioxidant capacity. This capacity has been interpreted as the mechanism which prevents oxidative stress caused by different stimuli[12,15,16]. The inhibition of oxidative stress generated in ischemic-reperfusion conditions in different experimental models including rat and rabbit has been explained similarly[17-19]. The in vitro antioxidant activity of CAPE has also been shown, since it reduced the levels of intracellular H2O2 and oxidized bases in DNA, probably by selective scavenging activity[20,21].
At present, evidence has accumulated that electrophiles are generated by direct and indirect carcinogens. In our hepatocarcinogenesis system, DEN metabolism produces free-radical metabolites. Additionally, cytochrome isoform 2E1 (CYP2E1) is the main cytochrome responsible of DEN metabolism, and capable of generating a prolonged burst of ROS near the site of substrate oxidation[22]. The products of these events are involved as cellular oxidant participants in neoplastic development[23]. Since oxidative stress during carcinogen metabolism participates in the initiation of hepatocarcinogenesis in the rat and CAPE counteracts oxidative stress, the present study was designed to examine the effect of CAPE on the initiation stage, evaluating its possible antioxidative mechanism of action in vivo in a medium-term hepatocarcinogenesis model and in vitro using a PHC.
MATERIALS AND METHODS
Animals
Male Wistar rats (180-200 g) obtained from the Production Unit of Experimental Laboratory Animals (UPEAL-Cinvestav, México D.F., México), had access to food (PMI Feeds Inc., Laboratories Diet) and water at all times; food cups were replenished with diet three times weekly. During treatment, the rats were transferred to the holding room, under controlled conditions of 12-h light/12-h dark cycle, 50% humidity, and 21°C temperature. All animals received human care according to the institutional guidelines for use of laboratory animals.
Experimental hepatocarcinogenic protocol
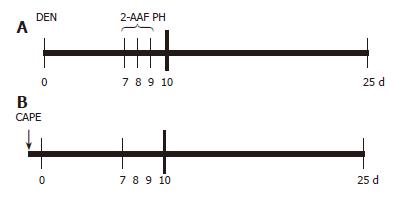
Animals were treated as described by Carrasco-Legleu et al[14]. Briefly, animals were administered 200 mg/kg DEN intraperitoneally; one week later, 2-AAF was orally administered with a stainless steel feeding tube at doses of 20 mg/kg per day on 3 consecutive days before partial hepatectomy (PH). Animals were sacrificed 25 d after DEN administration[24] and the livers excised, quickly frozen in liquid nitrogen and stored at -80°C until analysis. CAPE was obtained by esterification of caffeic acid with phenethyl alcohol as detailed previously[25], and dissolved in dimethylsulfoxide (DMSO) to evaluate its effect on initiation; it was administered IP at a dose of 20 mg/kg weight 12 h before DEN application (Figure 1).
Figure 1 CAPE administration protocol.
A: Carcinogenic treatment (CT): at time 0 the rats were treated with 200 mg/kg of DEN; on d 7, 8 and 9, 2-AAF was administered by gavage at doses of 20 mg/kg per day, a PH was performed at d 10 and rats were sacrificed at d 25; B: CAPE (↓) was administered 12 h before DEN.
Histochemistry of GGT
Histological liver sections of 15 μm thickness were obtained with a cryostat (Slee cryostat MTC, Germany) and stained for GGT activity according to Rutenburg et al[26]. Images of the GGT positive (GGT+) foci were captured with a digital camera (Color View 12, Soft Imaging System GmbH, Germany) and quantified with analysis software (AnalySIS Soft Imaging System GmbH, Germany). GGT+ areas larger than 0.01 mm2 were registered to avoid basal detection. Rat livers were sectioned in 5 mm slices, and 3 slices were randomly selected from each liver. Twenty histological sections were prepared from each liver slice and, randomly again, 4 out of the 20 were selected and prepared for computer analysis.
RT-PCR analysis
Total RNA of liver tissue was isolated with the method developed by Chomczynski and Sacchi[27]. It was treated with RNase-free DNase 1 (Boehringer-Mannheim, Germany) and one microgram of RNA was reverse transcribed into cDNA using the commercial kit (Gibco BRL/Life Technology, Inc., Gaithersburg, MD) at a final volume of 12.5 μL. α-Actin was used as an internal reference gene. The 2 primer sequences of rat GGT and αactin used were: 5'-CTCTGCATCTGGCTACCCAC-3' 5'-GGATGCTGGGTTGGAAGAGG-3' and 5'-CCAAGGCCAACCGCGAGAAGATGAC-3' 5'-GGTACATGGTGGTGCCGCCAGAC-3' (sense and antisense), respectively. The mixture was heated at 45°C for 30 min in a GeneAmp PCR System 2400 (Perkin-Elmer, Corp., CT). GGT and α-actin were amplified in 40 and 30 sequential cycles, the program was denaturation (94°C, 30 s), annealing (59°C, 45 s), and extension (70°C, 45 s). The PCR products were 418 bp and 586 bp, respectively. The amplified samples were visualized on 2% agarose gels, stained with ethidium bromide and captured with a digital camera (Kodak electrophoretic documentation and Analysis system 120).
Western blot analysis
For detection of GSTp in the cytosolic fraction, liver homogenates were prepared with lysis buffer (Tris-HCl 10 mmol/L pH 7.4, NaCl 150 mmol/L and phenylmethylsulfonyl fluoride (PMSF) 1 mmol/L) and were spun at 9000 r/min in a microcentrifuge at 4°C for 15 min. The cytoplasm protein concentration was measured with the bicinchoninic acid method[28]. SDS/PAGE was performed under reducing conditions on 12% polyacrylamide gels. Resolved proteins were transferred onto nitrocellulose sheets (Bio-Rad Lab., Hercules, CA), probed with a rabbit polyclonal anti-GSTp (Dako Corporation, Carpinteria, CA), treated with horseradish peroxidase-conjugated secondary antibody (Zymed, San Francisco, CA), detected by Enhanced Chemiluminescense detection reagent (Santa Cruz Biotechnology Inc., Santa Cruz, CA) and developed with Konica Film (Tokyo, Japan.). These membranes were then re-probed with anti-α-actin monoclonal antibody (Cinvestav, Mexico City) used as loading control.
Thiobarbituric acid reactive substances
Samples of frozen liver were homogenized in a buffer containing 10 mmol/L Tris, PMSF and NaCl, and protein was determined. Lipid peroxidation (LPX) was measured as TBARS, a widely used method, according to Buege and Aust[29]. Briefly, 600 mg of protein liver homogenates plus 300 μL of 0.4% thiobarbituric acid in 20% acetic acid, pH 3.0, were mixed and heated at 100°C for 45 min. Next, the samples were cooled, added with 200 μL of 1.2% KCl, 0.5 ml of 1:15 pyridine/butanol and centrifuged at 7500 r/min for 10 min. The resulting solution was determined at A532 nm, and using the extinction coefficient, E = 1.56 × 105, TBARS were expressed as nmoles of malonyldialdehyde as mg of proteins.
Unscheduled DNA synthesis assay in primary hepatocyte cultures
PHC were prepared from male Wistar rats (180-200 g) by the collagenase perfusion method as previously described[30], and three replicates of 8 × 105 cells were seeded onto 35 mm culture dishes. PHC were seeded in DMEM supplemented with 10% bovine fetal serum (HyClone, Laboratories Inc., Logan Utah) and 5 μg insulin/mL (Eli Lilly, México, DF) and placed in a 37°C humidified incubator in an atmosphere of 90% air/10% CO2.
After 2 h of incubation, the medium was removed and replaced by one ml of serum-free medium containing 10 μmol/L HU, 5 μCi of 3HdT from Amersham Life Science (Cleveland, OH) and 4 μL of DMSO as control. DEN, CAPE or MNNG were dispensed from 0.625 to a final concentration of 10 μmol/L per dish. Control dishes received the corresponding vehicle of CAPE and carcinogens. PHC were incubated for 4 h with their respective treatment. To account for variation in the number of cells between dishes, results were normalized as a function of DNA concentration. For this purpose DNA hydrolysate was obtained with the method by Leyva and Kelly[31] as described by Pérez-Carreón[32]. Incorporation of 3HdT in dpm per μg DNA was determined and the results were expressed, as percent of control incorporation.
Statistical analysis
Number and area of GGT+ AHF and GSTp protein expression of the group treated with CAPE were compared with the respective control group (CT plus vehicle). Results were analyzed by t. Differences were considered statistically significant at P < 0.05.
RESULTS
In vivo assays
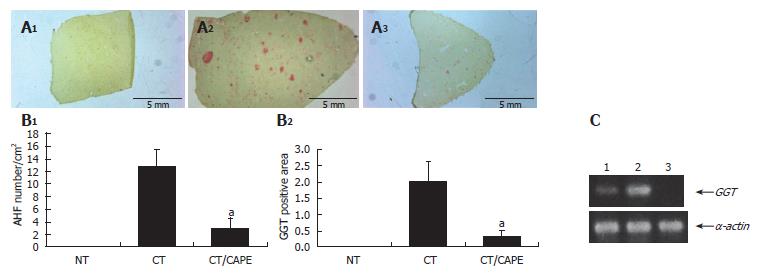
Protective effect of CAPE on hepatocarcinogenesis initiation: In this in vivo medium-term hepatocarcino-genesis assay, CAPE was tested on initiation. Rats which received the CT (DEN, 2-AAF and PH) were sacrificed 25 d after initiation. At this time, copious liver GGT+ AHF were histochemically detected, and their average-quantity was considered 100%. The CT animal group that received a single dose of CAPE before initiation clearly showed a decrease of GGT+ AHF formation, resembling that of the non-treated (NT) group (Figure 2A). Quantification of this phenomenon showed a drastic decrease in number and area of GGT+ AHF. CAPE had a protective effect of 84% and 91%, respectively (Figure 2B). Differences were considered significant when P < 0.05. We also evaluated the expression of transcripts of the GGT protein by the RT-PCR assay, and observed a decrease of transcripts due to the CAPE effect (Figure 2C)
Figure 2 Effect of CAPE on GGT expression.
A: GGT histochemically stained sections (1) non-treated group (NT), (2) carcinogenic treatment group (CT), (3) CT plus CAPE before DEN; B: CAPE effect on number/cm2 of AHF and percentage of GGT+ area/tissue. The differences of AHF number and area after the effect of CAPE in these groups were compared with the respective control group (CT + vehicle) and obtained values were significant by t with aP < 0.05. Twelve histological liver sections per rat from each treatment were randomly selected. NT (n = 3), CT (n = 4), CT plus CAPE (n = 7); C: The RT-PCR assay was used to determine GGT mRNA expression. L1: Control NT; L1: CT group; L1: CT plus CAPE. The series of the extreme right represents α-actin transcripts as control. This experiment was performed with a pool of total RNA isolated from 3 animals.
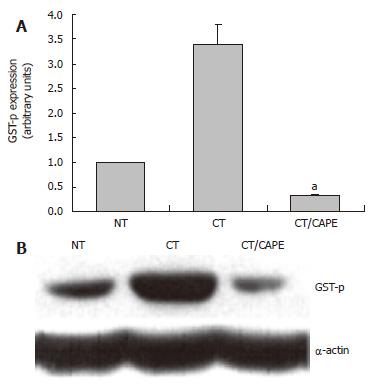
CAPE decreases the expression of the GSTp protein: The protective effects of CAPE with respect to GGT were toward its activity and its mRNA expression. GSTp is another marker related to the hepatocarcinogenesis model. We analyzed if CAPE modulates this marker at protein level. By Western blot we detected that CAPE drastically decreased the expression of the GSTp protein by 90% at d 25 (Figure 3). These results, in congruence with those related to GGT, showed the same protective profile of CAPE.
Figure 3 The effect of CAPE on GSTp protein expression.
Western blot was used to analyze the expression of the GSTP protein marker. A: Densitometric analysis of GSTP in: Control NT, CT and CT plus CAPE given before DEN. Results were statistically significant with aP < 0.05; B: Representative Western blot.
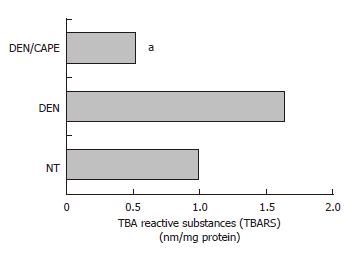
DEN-generated lipid peroxidation was decreased by CAPE: Since our laboratory previously has shown that LPX is an expression of oxidative stress in cells[33], we determined the TBARS as a global approach to measure LPX, and compared their levels obtained 24 h after administration of CAPE before initiation. Twelve hours after initiation with DEN, an increment of LPX of 68% above control level was observed (Figure 4). The administration of CAPE 12 h before administration of DEN importantly prevented full expression of LPX. CAPE decreased it to 52% with respect to the NT group of rats.
Figure 4 Effect of CAPE on LPX levels induced by DEN.
LPX was determined by detection of malonyldialdehyde concentration and expressed as TBARS. DEN increased TBARS levels detected at 12 h. CAPE administered 12 h before the initiator, clearly reduced the TBARS induced by DEN. Six hundred microgram of liver homogenates of each treated group were used, group NT, DEN (12 h after) and DEN plus CAPE (24 h after CAPE administration). Results were statistically significant with aP < 0.05.
In sum, these in vivo results showed that a single dose of CAPE, in addition to the protective effect on induction of GGT+ AHF, transcription of GGT, and expression of GSTp, produced a very important protective effect against oxidative stress.
In vitro assay
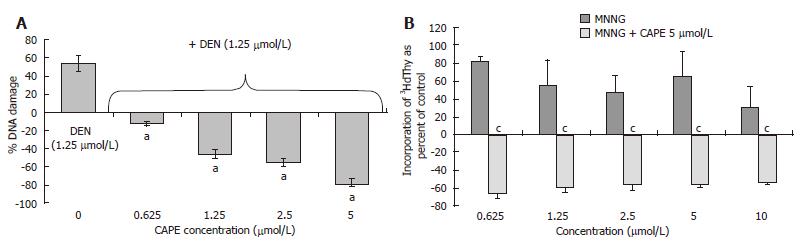
Protective effect of CAPE in vitro: The genotoxic alteration produced by carcinogens, one of the first measurable effects involved in initiation, can be analyzed with the UDS assay. This assay was used to test if CAPE protects against the genotoxic effect induced by 2 carcinogens with different mechanisms of activation: DEN, an indirect carcinogen that is activated by CYP2E1 and generates electrophylic species as well as ROS, and MNNG, a direct carcinogen that produces electrophylic proximal carcinogens by hydrolysis. The effect of CAPE on DEN-treated PHC of Wistar rats was analyzed; the incorporation of 3HdT in presence of vehicle (water or DMSO alone) was defined as control. Figure 5A shows that DEN at 1.25 μmol/L induced an increment of 3HdT incorporation of 60% above control level. CAPE alone at 1.25 and 2.5 μmol/L did not induce incorporation above control level (data not shown). The CAPE concentrations (0.625 to 5 μmol/L) abolished increments in 3HdT incorporation caused by 1.25 μmol/L DEN in a concentration-dependent form. We also analyzed if the action of CAPE is due to its free-radical scavenging abilities, MNNG being a mutagenic electrophylic species generator by hydrolysis. CAPE protected from the genotoxic damage to DNA produced by MNNG. The increment of 3HdT incorporation induced by MNNG was more pronounced than that induced by DEN. The maximum evoked response was 182% above control level at 0.625 μmol/L MNNG concentration. CAPE was tested at 5 μmol/L for its protective effect, and this concentration abolished the carcinogenic effect of 0.625 to 10 μmol/L MNNG and decreased 3HdT incorporation between 20 and 40% below control level (Figure 5B). CAPE inhibited the genotoxicity induced by both carcinogens tested, DEN and MNNG, suggesting that its protective mechanism is probably its capacity as scavenger of electrophylic molecules and not as inhibitor of carcinogenic activation in our system.
Figure 5 CAPE protects from genotoxic damage caused by DEN and MNNG in PHC.
A: DEN at 1.25 μmol/L concentration induced 3HdT incorporation and this dose was tested with several concentrations of CAPE (0.625, 1.25, 2.5, 5 and 10 μmol/L). CAPE decreased DEN genotoxic damage to levels even below the control; B: Curve concentration of MNNG from 0.625-10 μmol/L. One dose of 5 μmol/L of CAPE protects from genotoxic damage caused by MNNG in PHC. Data incorporation of 3HdT was estimated as dpm/mg of DNA and results are expressed as percent of control samples, the zero data represent 3HdT incorporation of basal level of control group. Results were obtained from 3 replicates of at least 3 animals. aP < 0.05 vs DEN only; cP < 0.05 vs MNNG only.
DISCUSSION
Present results clearly show a protective effect of CAPE when a single dose was given before initiation in the rat hepatocarcinogenesis model. CAPE decreased the induction of both, area and number of GGT+ AHF, GGT transcripts and of the GSTp protein. Our results showed a protective effect against induction of preneoplastic lesions and are an addition to previously reported data[11-14]. These effects are in accordance with the effective chemoprotective antioxidant activity reported for CAPE[34,35] and are further supported by in vitro evaluation, in mouse epidermal cells, where CAPE was found to be one of the four better chemo-protective agents among 25 well-known substances with this activity[36].
In this context, it has been postulated that overproduction of ROS and oxidative DNA damage play a major role in cancer development. Increased concentrations of active oxygen, organic peroxides and radicals can promote initiated cells to neoplastic growth, inducing alterations in DNA structure or producing epigenetic mechanisms[23,37]. This justified the investigation of the effect of CAPE during the early stages of liver carcinogenesis, where we had specifically demonstrated the obligated participation of oxidative stress in the production of preneoplastic lesions during initiation in our medium-term hepatocarcinogenesis model. We had previously shown that ROS importantly participate in our model of hepatocarcinogenesis, and quercetin, an antioxidant molecule, prevents the appearance of AHF and drastically decreases LPX induced by DEN[33]. These results are in accord with observations in another system where CAPE decreased LPX levels[38]. Our proposition is that many, if not all, protective effects of CAPE have a common mechanism, which is its anti-oxidative and free-radical scavenging abilities that involve several cellular pathways.
Present in vitro UDS assay results in PHC with two carcinogens, one indirect and the other a direct, support our hypothesis. The protective effect of CAPE against DEN involves at least two possible explanations: (1) CAPE intercepts electrophylic metabolites and ROS formed through the CYP2E1 activity and, in this way, blocks their interaction with DNA and prevents induction of DNA damage, or (2), CAPE inhibits CYP2E1, as is the case of the chemoprotector molecule, sulforophane[39], and prevents formation of DEN electrophylic metabolites, which prevents UDS. Prevention of MNNG-induced UDS could be explained only by the CAPE anti-oxidative and free-radical scavenging ability since MNNG electrophylic metabolites are generated without participation of cytochrome enzymes and ROS generation. Moreover, it has been shown that tea polyphenols prevent MNNG induction of gastric cancer[40]. A stronger evidence in this context has been obtained in Swiss mice treated with MNNG; garlic, tomato or neem leaf extracts prevent the induction of micronuclei and the increment of LPX in bone marrow, as well as enhanced GSH-dependent antioxidant activities[41,42]. Even though, in the case of DEN, we cannot discard the possibility that the mechanism by which CAPE prevents AHF is via CYP2E1 inhibition, the fact that the MNNG genotoxic effect is prevented by CAPE, supports the notion that the protective mechanism of CAPE is due to its anti-oxidative and free-radical scavenging activities. This proposition is also supported by results in a cell-free assay used to evaluate antioxidant and free-radical scavenging activities of several chemopreventive agents[43]. In these studies, CAPE revealed the third highest antioxidant activity after caffeic acid and α-tocopherol, and the second highest free-radical scavenging activity after rosmarinic acid. This evidence together with previous studies in models that show that the protective effect of CAPE against cancer in rodents is related to its anti-oxidant activity support our proposal[11-13,44].
The chemoprotective effect of CAPE in our medium-term model of hepatocarcinogenesis diminished ggt mRNA expression, drastically decreases the number and area of GGT+ AHF, and the GSTP protein expression; it abolished LPX and, in addition, it prevented the UDS induced by DEN or by MNNG in PHC. In conclusion, we suggest that the protective effects of CAPE at initiation as shown in vivo and in vitro are due to its anti-genotoxic, anti-oxidative and free-radical scavenging abilities.