Published online Nov 7, 2006. doi: 10.3748/wjg.v12.i41.6727
Revised: July 20, 2006
Accepted: July 29, 2006
Published online: November 7, 2006
We describe a rare case of HCV-related recurrent multiple hepatocellular carcinoma (HCC) metastasizing to the skull base involving multiple cranial nerves in a 50-year-old woman. The patient presented with symptoms of ptosis, fixation of the right eyeball, and left abducens palsy, indicating disturbances of the right oculomotor and trochlear nerves and bilateral abducens nerves. Brain contrast-enhanced computed tomography (CT) revealed an ill-defined mass with abnormal enhancement around the sella turcica. Brain magnetic resonance imaging (MRI) disclosed that the mass involved the clivus, cavernous sinus, and petrous apex. On contrast-enhanced MRI with gadolinium-chelated contrast medium, the mass showed inhomogeneous intermediate enhancement. The diagnosis of metastatic HCC to the skull base was made on the basis of neurological findings and imaging studies including CT and MRI, without histological examinations. Further studies may provide insights into various methods for diagnosing HCC metastasizing to the craniospinal area.
- Citation: Kim SR, Kanda F, Kobessho H, Sugimoto K, Matsuoka T, Kudo M, Hayashi Y. Hepatocellular carcinoma metastasizing to the skull base involving multiple cranial nerves. World J Gastroenterol 2006; 12(41): 6727-6729
- URL: https://www.wjgnet.com/1007-9327/full/v12/i41/6727.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i41.6727
Hepatocellular carcinoma (HCC), the most common primary tumor of the liver, is estimated to cause more than a quarter of a million deaths throughout the world each year. Metastasis is one of the most significant factors affecting prognosis. In HCC, intrahepatic metastasis is frequent, and extrahepatic metastasis to the lung, bone and adrenal glands is detected sometimes, while cranial nerves are not likely to be involved in metastatic HCC. We describe a case of a 50-year-old woman with symptoms of ptosis, fixation of the right eyeball, and left abducens palsy caused by HCC metastasizing to the skull base, diagnosed through neurological findings and imaging studies.
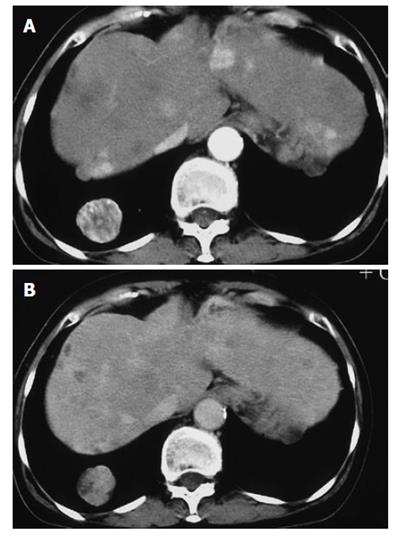
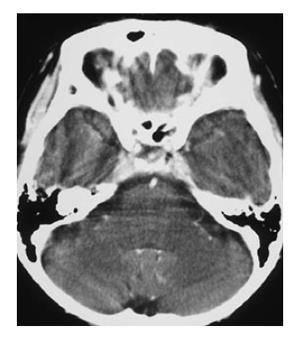
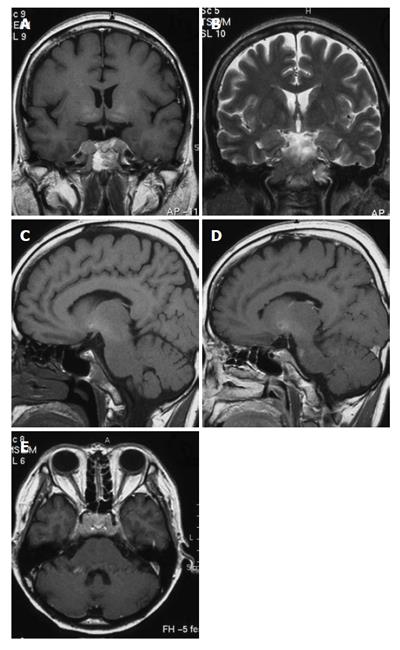
A 50-year-old woman was admitted to Kobe Asahi Hospital in October 2002 with symptoms of ptosis, fixation of the right eyeball and left abducens palsy. Since 1989, multiple HCCs which had occurred repeatedly with the background of type C liver cirrhosis were treated more than ten times with percutaneous ethanol injection (PEI) and transcatheter embolization (TAE). Neurological findings were normal except for disturbances of the right oculomotor and trochlear nerves and bilateral abducens nerves. Laboratory studies on admission disclosed the following abnormal values: aspartate aminotransferase 84 kIU/L (normal 5-35 kIU/L), alanine aminotransferase 38 IU/L (normal 4-43 IU/L), hemoglobin 103 g/L (normal 113-152 g/L), platelets 14.7 × 1010/L (normal 13.0-36.9 × 1010/L), thymol turbidity 5.0 × 106 IU/L (normal 0-4.0 × 106 IU/L), zinc surface turbidity 15.1 × 106 IU/L (normal 2.0-12.0 × 106 IU/L), and γ-globulin 266 g/dL (normal 105-203 g/dL). The levels of tumor markers were 184 mg/L (normal 0-0.02 mg/L) for alfa-fetoprotein (AFP), 6.4% (normal 0%-10%) for lens culinaris agglutinin A-reactive fraction of AFP (AFP L3) and 22.3 kAU/L (normal 0-0.04 kAU/L) for protein induced by vitamin K absence (PIVKA II). Incremental dynamic computed tomography (CT) revealed multiple HCC nodules in both lobes (Figure 1). Chest X-ray showed extrahepatic metastases to both lungs; however, imaging studies did not show metastases to the bone or adrenal gland. Brain contrast-enhanced CT (Figure 2) revealed an ill-defined mass with abnormal enhancement around the sella turcica. On unenhanced brain magnetic resonance imaging (MRI) the mass was shown to involve the clivus, cavernous sinus, and petrous apex. Inhomogeneous intermediate enhancement of the mass was confirmed by contrast-enhanced MRI with gadolinium-chelated contrast medium. Meningeal thickening from the posterior of the sella turcica to the clivus and dilation of the superior carotid vein were observed (Figure 3).
The third, fourth, and sixth cranial nerves may be involved in lesions affecting their nuclei or fibers of efferent in the pons or the mesencephalon, or in the course of nerves through the subarachnoid space, the cavernous sinus, and the superior orbital fissure. Different syndromes occur with lesions at each of these sites. From neurological findings such as ptosis, fixation of the right eyeball, and left abducens palsy, we suspected the involvement of the multiple cranial nerves. Brain CT and MRI with contrast medium revealed abnormal enhancement around the sella turcica, and brain MRI revealed that the mass involved the clivus, cavernous sinus, and petrous apex, therefore we made the definite diagnosis of HCC metastasizing to the skull base.
Metastasis appears to be a late event in the natural history of HCC, and most patients die of liver failure attributed to liver replacement by the tumor. Extrahepatic metastases are commonly found at autopsy in over half of the cases[1-3], the lung, being the most frequent (approximately 50%) and the regional lymph nodes often being the sites of metastasis. In addition, bone metastases are common, and patients may show a bony metastasis as the initial presentation of occult HCC. Adrenal metastases are found in up to 15% of autopsies, and some cases of pedunculated HCC have been reported as actual metastasis to the right adrenal gland from a nearby tumor. Conversely, the brain is less common metastatic sites. Incidental extrahepatic lesion at such more uncommon site is less confidently viewed as potential metastases when metastatic disease is not detected at the more common sites (the lungs, lymph nodes, and bone). According to neurological findings and imaging studies of brain CT and MRI, our case was confirmed as metastatic HCC to the skull base involving multiple cranial nerves including the third, fourth, and sixth.
The central nervous system is an unusual site of metastatic HCC[4-12]. Seven cases of HCC presenting as cranial metastasis without obvious hepatic involvement have been described: metastatic spread of HCC to the cerebrum in one case, and to the cranium in six cases[8], all manifesting mildly abnormal liver function when first evaluated, leading to the conclusion that in cranial metastasis of unknown origin in a geographical area where HCC is a common disease, HCC should be considered in the differential diagnosis[8]. In Japan as in Taiwan, where HCC is a common disease, however, metastatic HCC to the skull base involving multiple cranial nerves has not been reported so far, except for a case of skull metastasis of HCC associated with acute epidural hematoma[9].
Before 1990, the diagnosis of metastatic HCC to the craniospinal area was confirmed by histological examinations of biopsy, surgical and autopsy specimens. Recently diagnosis is reached by neurological findings and imaging studies, such as CT and MRI because of improvements in these modalities. With the recent advances in the nonsurgical treatment of HCC, such as percutaneous ablation including percutaneous ethanol injection therapy (PEIT), radiofrequency ablation (RFA) and transcatheter embolization, longer-term survival has been attained, even in cases of advanced HCC compared with older autopsy populations. This may be due to current treatment regimens of chemotherapy or chemoembolization, or both, that result in the longer survival of patients diagnosed with HCC, as was in our case. Although HCC metastasizing to the skull base involving multiple cranial nerves is very rare, clinicians need to be vigilant and would do well to conduct imaging studies such as CT and MRI, when neurological findings reveal suspected cranial nerve involvement.
S- Editor Wang GP L- Editor Zhu LH E- Editor Ma WH
| 1. | Nakashima T, Okuda K, Kojiro M, Jimi A, Yamaguchi R, Sakamoto K, Ikari T. Pathology of hepatocellular carcinoma in Japan. 232 Consecutive cases autopsied in ten years. Cancer. 1983;51:863-877. [RCA] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 2. | Kojiro M, Nakashima T. Pathology of hepatocellular carcinoma. Neoplasms of the liver. Tokyo: Springer-Verlag 1987; 81-104. [DOI] [Full Text] |
| 3. | Yuki K, Hirohashi S, Sakamoto M, Kanai T, Shimosato Y. Growth and spread of hepatocellular carcinoma. A review of 240 consecutive autopsy cases. Cancer. 1990;66:2174-2179. [RCA] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 4. | Ihde DC, Sherlock P, Winawer SJ, Fortner JG. Clinical manifestations of hepatoma. A review of 6 years' experience at a cancer hospital. Am J Med. 1974;56:83-91. [RCA] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 117] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Chang YC, Chen RC. Craniospinal and cerebral metastasis of primary hepatomas: a report of 7 cases. Taiwan Yixuehui Zazhi. 1979;78:594-604. |
| 6. | Collomb H, Sankalé M, Dumas M, Ancelle JP. [Neurologic forms of primary liver cancer (clinical and electroencephalographic study)]. Bull Soc Med Afr Noire Lang Fr. 1968;13:577-585. [PubMed] |
| 7. | Zubler MA, Rivera R, Lane M. Hepatoma presenting as a retro-orbital metastasis. Cancer. 1981;48:1883-1885. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Lee JP. Hepatoma presenting as craniospinal metastasis: analysis of sixteen cases. J Neurol Neurosurg Psychiatry. 1992;55:1037-1039. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Hayashi K, Matsuo T, Kurihara M, Daikoku M, Kitange G, Shibata S. Skull metastasis of hepatocellular carcinoma associated with acute epidural hematoma: a case report. Surg Neurol. 2000;53:379-382. [RCA] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Asahara T, Yano M, Fukuda S, Fukuda T, Nakahara H, Katayama K, Itamoto T, Dohi K, Nakanishi T, Kitamoto M. Brain metastasis from hepatocellular carcinoma after radical hepatectomy. Hiroshima J Med Sci. 1999;48:91-94. [PubMed] |
| 11. | Lee JP, Lee ST. Hepatocellular carcinoma presenting as intracranial metastasis. Surg Neurol. 1988;30:316-320. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Phadke JG, Hughes RC. Hepatocellular carcinoma with cranial metastasis and hyperglobulinaemia. J Neurol Neurosurg Psychiatry. 1981;44:1171-1172. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |