Published online Nov 7, 2006. doi: 10.3748/wjg.v12.i41.6678
Revised: September 3, 2006
Accepted: September 6, 2006
Published online: November 7, 2006
AIM: To utilize transcatheter arterial steroid injection therapy (TASIT) via the hepatic artery to reduce hepatic macrophage activity in patients with severe acute hepatic failure.
METHODS: Thirty-four patients with severe acute hepatic failure were admitted to our hospital between June 2002 to June 2006 providing for the possibility of liver transplantation (LT). Seventeen patients were treated using traditional liver supportive procedures, and the other 17 patients additionally underwent TASIT with 1000 mg methylprednisolone per day for 3 continuous days.
RESULTS: Of the 17 patients who received TASIT, 13 were cured without any complications, 2 died, and 2 underwent LT. Of the 17 patients who did not receive TASIT, 4 were self-limiting, 7 died, and 6 underwent LT. Univariate logistic analysis revealed that ascites, serum albumin, prothrombin time, platelet count, and TASIT were significant variables for predicating the prognosis. Multivariate logistic regression analysis using stepwise variable selection showed that prothrombin time, platelet count, and TASIT were independent predictive factors.
CONCLUSION: TASIT might effectively prevent the progression of severe acute hepatic failure to a fatal stage of fulminant liver failure.
- Citation: Kotoh K, Enjoji M, Nakamuta M, Yoshimoto T, Kohjima M, Morizono S, Yamashita S, Horikawa Y, Yoshimitsu K, Tajima T, Asayama Y, Ishigami K, Hirakawa M. Arterial steroid injection therapy can inhibit the progression of severe acute hepatic failure toward fulminant liver failure. World J Gastroenterol 2006; 12(41): 6678-6682
- URL: https://www.wjgnet.com/1007-9327/full/v12/i41/6678.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i41.6678
Although it is accepted that severe acute hepatic failure is self-limiting in most patients, it progresses to fulminant liver failure with a high mortality rate in some patients[1]. Liver transplantation (LT) is the only effective treatment for patients with fulminant liver failure[2]. It seems that early treatment for severe acute hepatic failure could effectively reduce the mortality, but it is challenged with several reasons. First, it is difficult to determine the likelihood of progression from severe acute hepatic failure to fulminant liver failure. Although studies have proposed several predictive parameters, such as coagulopathy, jaundice, etiology, and aging[3-7], a reliable and widely accepted procedure for distinguishing self-limiting cases from fatal cases has not been established. Second, even if it might be possible to predict the prognosis of severe acute liver failure, no effective procedures exist for preventing the progression from severe acute hepatic failure to fulminant liver failure. Plasma exchange and hemodiafiltration have been used as artificial liver support systems, but they are only partially effective[8-11].
The development of methods for inhibiting the progression of severe acute hepatic failure to fulminant liver failure has been hampered by a lack of understanding of the mechanisms of fulminant liver failure. The reason why the condition is fatal in some patients but remits spontaneously in others even among cases sharing a common etiology remains unclear. Several recent studies indicate that activated macrophages in the liver might play a key role in the development of fulminant liver failure[12-15]. If this hypothesis is correct, then suppressing intrahepatic macrophage activity might be a useful treatment.
It is well known that macrophage activity is suppressed by corticosteroids[16,17], which have been used in the past as a treatment for severe acute hepatic failure, but their effectiveness has not been definitively established[18-20]. We speculated that the disappointing results might be due to an insufficient concentration of corticosteroids in the hepatic circulation, and therefore injected corticosteroids via the hepatic artery as a treatment for patients with severe acute hepatic failure. Selective arterial injection has been used for the treatment of hepatocellular carcinoma[21,22]. Infusion of 5-flourouracil or cisplatin via the hepatic artery is effective while peripheral intravenous injection via the hepatic artery is not effective against hepatocellular carcinoma. In this study, patients with severe acute hepatic failure were enrolled and prognostic factors, including arterial steroid injection therapy, were analyzed.
The patients enrolled between June 2002 to June 2006 in this study were admitted to our hospital with a diagnosis of severe acute hepatic failure and fulfilled at least one of the following criteria: progressive and sustained prothrombin time (PT) (PT-international normalized ratio (INR) > 1.5 for more than 3 d), presence of ascites, and presence of hepatic encephalopathy. When obvious liver atrophy and hepatic coma were observed on admission, the patient was immediately prepared for liver transplantation and excluded from the study. Transcatheter arterial steroid injection therapy (TASIT) was administered with the consent of the patient or in cases of severe encephalopathy greater than gradeII, with the written informed consent of the patient’s family. When consent was not obtained, traditional supporting treatments were continued. Therefore, this study was not a randomized trial. Plasma exchange was repeated to keep PT-INR lower than 1.7, and hemodiafiltration was performed if necessary. Regardless of the method of treatment, LT was implemented in cases whose liver insufficiency progressed when an appropriate donor could be found.
A 5-frame catheter was inserted from the right femoral artery to the common hepatic artery. Before the steroid injection, angiography was performed to determine whether an anomaly of the hepatic artery existed. When no replaced hepatic artery was found, the tip of the catheter was set at the proper hepatic artery. When the liver blood was supplied from two hepatic arteries, the branch that showed the widest feeding area was selected for positioning of the catheter. After insertion of the catheter, 1000 mg methylprednisolone was infused for 2 h per day. The arterial steroid injections were continued for 3 d, and the catheter was removed just after injection on the third day. When a bleeding tendency was observed during this protocol, once-daily plasma exchange was added to the regimen.
The details of the informed consent statement and the arterial steroid injection protocol were approved by the Ethics Committee of Kyushu University.
Differences in clinical backgrounds, symptoms, and laboratory data between patients who did or did not receive TASIT were analyzed using χ2 test and Student t-test. Factors potentially associated with the prognosis of severe acute hepatic failure were analyzed by univariate analysis followed by multivariate logistic regression analysis using stepwise variable selection. Factors with a P value > 0.10 were removed from the multivariate analysis.
Between June 2002 to June 2006, 34 patients (21 males and 13 females) were enrolled in this study. Seventeen patients gave their consent to receive TASIT and underwent the treatment. The characteristics of the patients on admission are shown in Table 1. The overall outcome was as follows: 17 patients were conservatively cured, 9 died, and 8 underwent LT. Since it is difficult to find donors for orthotopic transplantation in Japan, living donor LT was performed if necessary. All patients who underwent LT survived the procedure. Pathological examination of liver samples resected during LT showed that the injured liver was markedly atrophic and wholly necrotized in each case, indicating that the cases selected for LT were correctly identified as candidates for LT.
| Arterial steroid injection therapy | ||||
| Yes | No | Total | P | |
| n | 17 | 17 | 34 | |
| Sex (male/female) | 11/6 | 10/7 | 21/13 | 0.7242 |
| Age (yr) | 45.1 ± 16.9 | 44.8 ± 19.0 | 45.0 ± 17.7 | 0.9623 |
| Ascites (none/present) | 12/5 | 7/10 | 19/15 | 0.0842 |
| Encephalopathy | 5/4/3/5 | 11/0/5/1 | 16/4/8/6 | 0.0242 |
| (0/1/2/3) | ||||
| T.bilirubin (mg/dL) | 9.04 ± 9.21 | 14.48 ± 9.62 | 11.76 ± 9.67 | 0.1014 |
| D.bilirubin (mg/dL) | 6.15 ± 6.73 | 9.08 ± 6.71 | 7.61 ± 6.79 | 0.2131 |
| D/T bilirubin ratio | 0.66 ± 0.06 | 0.61 ± 0.12 | 0.64 ± 0.10 | 0.1785 |
| Albumin (g/dL) | 3.55 ± 0.46 | 3.25 ± 0.32 | 3.40 ± 0.42 | 0.0362 |
| ChE (mg/dL) | 111.7 ± 40.5 | 95.4 ± 31.3 | 103.8 ± 36.7 | 0.2047 |
| AST (U/L) | 5817.5 ± 4223.6 | 3203.71 ± 2829.2 | 4510.62 ± 3780.2 | 0.0530 |
| ALT (U/L) | 4056.7 ± 2427.3 | 3643.71 ± 2677.1 | 3850.21 ± 2525.0 | 0.6407 |
| AST/ALT ratio | 1.58 ± 1.16 | 0.99 ± 0.68 | 1.28 ± 0.98 | 0.0833 |
| ALP (U/L) | 539.5 ± 258.3 | 517.8 ± 178.0 | 528.6 ± 218.1 | 0.7775 |
| γ-GTP (U/L) | 336.4 ± 351.2 | 182.0 ± 182.0 | 261.5 ± 288.7 | 0.1228 |
| LDH (U/L) | 4349.3 ± 4479.0 | 1343.3 ± 2553.1 | 2846.3 ± 3900.6 | 0.0238 |
| PT-INR | 2.50 ± 1.60 | 2.93 ± 1.92 | 2.71 ± 1.76 | 0.4890 |
| Platelet (× 104/μL) | 13.9 ± 5.5 | 11.8 ± 4.2 | 12.8 ± 4.9 | 0.2121 |
| AFP (ng/mL) | 16.7 ± 27.2 | 29.6 ± 43.8 | 23.2 ± 36.5 | 0.3125 |
| NH3 (μg/dL) | 90.5 ± 87.0 | 63.6 ± 32.2 | 77.1 ± 66.1 | 0.2451 |
| BUN (mg/dL) | 14.1 ± 10.9 | 14.8 ± 18.6 | 14.4 ± 15.0 | 0.8850 |
| Creatinine (mg/dl) | 1.20 ± 1.30 | 1.16 ± 1.24 | 1.18 ± 1.25 | 0.9210 |
| Outcome | 13/2/2 | 4/7/6 | 17/9/8 | 0.0085 |
| (Survival/death/LT) | ||||
Patient backgrounds on admission did not differ significantly except for the encephalopathy-grading, serum albumin and LDH concentration between patients who did or did not undergo TASIT. In patients who did not receive TASIT, 4 were conservatively cured, 7 died, and 6 underwent LT. In patients who received TASIT, 13 were conservatively cured, 2 died, and 2 underwent LT. All deaths among patients who did not receive TASIT were due to progression to liver failure, while in the TASIT group, one patient died of liver failure and the other died of pneumonia seven days after initial recovery from hepatic insufficiency.
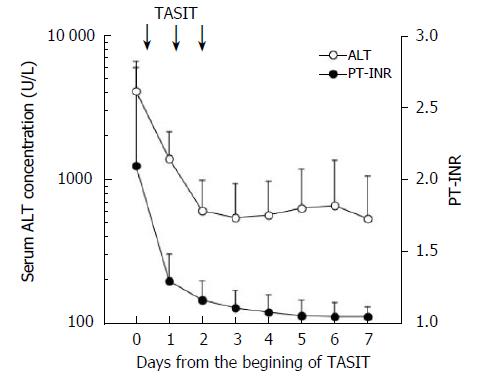
Rapid improvement of liver function was observed in the conservatively cured 13 survivors who received TASIT during the 3-d protocol. Encephalopathy was observed in 9 of the conservatively cured survivors on admission, and completely disappeared on the second day of the TASIT protocol. As shown in Figure 1, serum ALT concentrations decreased remarkably and coagulopathy rapidly improved. Serum ALT concentrations rebounded slightly for a few days in most of the survivors just after the end of the TASIT protocol, and subsequently decreased and normalized within 3 wk after TASIT in all but 1 patient. Even in 1 exceptional case showing prolonged serum ALT elevation after TASIT, the ALT level was much lower compared to that on admission and PT stayed within the normal range. The hepatic function of the patient normalized five weeks after TASIT, but the etiology remained unknown.
To determine prognostic factors, patients were assigned to 2 groups based on their clinical outcomes: one group consisted of conservatively cured survivors who did not receive LT and the other included fatal cases and LT recipients who were judged to have a poor prognosis without LT, based on pathological findings of the injured liver. Univariate logistic analysis was performed for age, sex, etiology of hepatitis, administration of TASIT, laboratory data, and symptoms on admission (Table 2). Among these parameters, presence of ascites, concentration of serum albumin (> 3.3 g/dL), PT-INR (> 2.0), platelet count (> 12 × 104/μL), and TASIT were significant factors (P < 0.05). Stepwise multiple regression analysis was performed using variables with P values ≤ 0.1, which revealed that PT-INR, platelet count, and TASIT were independent prognostic factors (Table 3). The P value of the prediction equation was < 0.0001.
| Variables | Estimate | P | Lower 95% | Upper 95% | Odds ratio |
| Age (50 vs ≤ 50 yr) | -0.6061 | 0.0912 | -0.0802 | 1.3415 | 3.3608 |
| Sex (male vs female) | 0.1247 | 0.7244 | -0.5708 | 0.8322 | 1.2833 |
| Ascites (none vs present) | 1.2079 | 0.0036 | 0.4465 | 2.1030 | 11.1997 |
| Encephalopathy (none vs present) | 0 | 1.0000 | -0.6799 | 0.6799 | 1 |
| T.Bil (< 8 mg/dL vs ≥ 8) | 0.4745 | 0.4930 | -0.8767 | 1.8627 | 1.6071 |
| D/T. Bil ratio (> 0.65 vs ≤ 0.65) | 0.4884 | 0.4870 | -0.8825 | 1.9022 | 1.6296 |
| Albumin (> 3.3 vs ≤ 3.3 g/dL) | 1.4816 | 0.0440 | 0.0865 | 3.0029 | 4.4000 |
| ChE (> 100 vs ≤ 100 mg/dL) | 0.8574 | 0.2306 | -0.5244 | 2.3092 | 2.3571 |
| AST (> 3500 vs ≤ 3500 U/L) | 0.4745 | 0.4930 | -0.8767 | 1.8627 | 1.6071 |
| ALT (> 4000 vs ≤ 4000 U/L) | 0.9628 | 0.1735 | -0.3993 | 2.4025 | 2.6190 |
| AST/ALT ratio (> 1 vs ≤ 1) | 0.2356 | 0.7317 | -1.1151 | 1.6042 | 1.2656 |
| LDH (> 600 vs ≤ 600 U/L) | 1.2122 | 0.0912 | -0.1603 | 2.6831 | 3.3608 |
| PT-INR (> 2 vs ≤ 2) | 1.4816 | 0.0440 | 0.0865 | 3.0029 | 20.1446 |
| Platelet (>12 × 104/μL vs ≤ 12 × 104) | 1.7509 | 0.0200 | 0.3335 | 3.3197 | 5.7600 |
| AFP (< 5 vs ≥ 5 ng/mL) | 0.2356 | 0.7317 | -1.1151 | 1.4616 | 1.2656 |
| NH3 (< 55 vs ≥ 55 μg/mL) | 0.2356 | 0.7317 | -1.1151 | 1.6042 | 1.2656 |
| BUN (< 10 vs ≥ 10 mg/dL) | 0.2356 | 0.7317 | -1.1151 | 1.6042 | 1.2656 |
| Etiology | |||||
| HBV (vs HAV) | 0.3269 | 0.5761 | -0.8196 | 1.5405 | 1.9230 |
| Others (vs HAV) | 0.1446 | 0.7567 | -0.7763 | 1.0744 | 1.3354 |
| TASIT (yes vs no) | 1.1787 | 0.0036 | 0.4311 | 2.0387 | 10.5624 |
| Variables | Estimate | P | Lower 95% | Upper 95% | Odds ratio |
| TASIT (done) | 1.6535 | 0.0076 | 0.6220 | 3.2312 | 27.3009 |
| Platelet (< 12 × 104/μL) | 2.6871 | 0.0261 | 0.6450 | 5.7805 | 14.6895 |
| PT-INR (< 1.0) | 1.8711 | 0.0687 | -0.0119 | 4.1505 | 6.4953 |
It is difficult to predict whether an individual patient with acute hepatitis develops fulminant liver failure because the condition often resolves spontaneously without any aftereffects. Severe acute hepatic failure has been generally defined as a clinical continuum between acute hepatitis without serious coagulopathy (PT > 50%) and acute liver disease complicated by clinical encephalopathy[1], which is considered a transient stage giving warning of fulminant liver failure. However, according to this definition of severe acute hepatic failure, two-thirds of patients spontaneously recover without developing encephalopathy[3]. Since such a high rate of conservative recovery is inappropriate for evaluating the effects of treatments designed to prevent progression of severe acute hepatic failure to fulminant liver failure, we established a more stringent criterion as a pre-stage of fulminant liver failure, namely acute severe hepatitis that has any of the following conditions: progressive and sustained PT (PT-INR > 1.5 more than 3 d), presence of ascites, and presence of hepatic encephalopathy. We speculated that PT at a point could not predict progression of severe acute hepatic failure to fulminant liver failure since a transient severe decrease of PT-INR is often observed even in self-limited acute hepatitis. Therefore, coagulopathy should be present for a specific period of time to enroll the patients close to fulminant liver failure. In this study, only 4 of 17 patients (23.5%) who were not treated with TASIT were conservatively cured. The remaining 13 patients died or underwent LT, which led to a markedly poorer prognosis than that reported in previous investigations in patients with severe acute hepatic failure[3,6]. The fatal outcomes indicate that our method for assessment of severe acute hepatic failure can identify patients who have a high risk of developing fulminant liver failure.
Multivariate logistic analysis revealed that TASIT could effectively prevent the progression of severe acute hepatic failure to fulminant liver failure. In light of past reports showing that corticosteroids have no effect on severe acute hepatic failure[18-20], we suggest that the effectiveness of TASIT may be due to the high concentrations of corticosteroids achieved in the hepatic circulation. However, we have not yet elucidated the mechanisms by which high concentrations of corticosteroids affect hepatic macrophages or damage hepatocytes. Furthermore, the role of macrophages in severe acute hepatic failure needs to be further clarified.
We believe that our protocol is safe and effective, but the ideal dose and duration of corticosteroid treatment should be verified. Although excessive use of corticosteroids generally induces infections that would hinder LT, 2 patients in this study who underwent LT after TASIT recovered without any complications, suggesting that our protocol does not seem to influence the outcome of LT. However, lower corticosteroid doses might be sufficient to prevent the progression of the disease. Randomized, controlled studies are needed to confirm this possibility.
Among the patients who received TASIT, 4 were not conservatively cured, 2 underwent LT and 2 died without LT. Since one of the deaths resulted from pneumonia after recovery from hepatic failure, TASIT was found to be ineffective for severe acute hepatic failure in 3 patients. A common finding in the 3 patients was rapid atrophy of the liver, showing an obvious decrease in liver volume within 24 h of admission, indicating that TASIT is not effective for patients who are at the later stage of fulminant liver failure. Therefore, TASIT should be performed when possible warning signs of the progression of severe acute hepatic failure to fulminant liver failure are observed. However, early administration of TASIT would involve patients who might otherwise recover following traditional supportive measures. Thus it is possible that some of the patients treated with TASIT in this study may have been cured without the treatment. Nonetheless, considering the results of multivariate logistic analyses and the high cost of LT, we believe that TASIT is advantageous over the traditional liver supportive procedures.
In this study, we demonstrated a new treatment strategy for preventing the progression of severe acute liver failure to fulminant liver failure. Our results indicate that TASIT is a safe and effective treatment for severe acute hepatic failure. However, its effect is insufficient in patients showing marked and rapid progression of liver atrophy. Therefore, the option of LT should be considered simultaneously. Randomized, controlled trials are needed to further evaluate this treatment strategy.
S- Editor Wang J L- Editor Wang XL E- Editor Bai SH
| 1. | Bernuau J, Benhamou J. Fulminant and subfulminant liver failure. Oxford textbook of clinical hepatology. Oxford: OXFORD UNIVERSITY press 1999; 1341-1372. |
| 2. | Ascher NL, Lake JR, Emond JC, Roberts JP. Liver transplantation for fulminant hepatic failure. Arch Surg. 1993;128:677-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 112] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Takikawa Y, Endo R, Suzuki K, Fujiwara K, Omata M. Prediction of hepatic encephalopathy development in patients with severe acute hepatitis. Dig Dis Sci. 2006;51:359-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Miyake Y, Sakaguchi K, Iwasaki Y, Ikeda H, Makino Y, Kobashi H, Araki Y, Ando M, Kita K, Shiratori Y. New prognostic scoring model for liver transplantation in patients with non-acetaminophen-related fulminant hepatic failure. Transplantation. 2005;80:930-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Dhiman RK, Seth AK, Jain S, Chawla YK, Dilawari JB. Prognostic evaluation of early indicators in fulminant hepatic failure by multivariate analysis. Dig Dis Sci. 1998;43:1311-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Elinav E, Ben-Dov I, Hai-Am E, Ackerman Z, Ofran Y. The predictive value of admission and follow up factor V and VII levels in patients with acute hepatitis and coagulopathy. J Hepatol. 2005;42:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Dabos KJ, Newsome PN, Parkinson JA, Mohammed HH, Sadler IH, Plevris JN, Hayes PC. Biochemical prognostic markers of outcome in non-paracetamol-induced fulminant hepatic failure. Transplantation. 2004;77:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Davenport A, Will EJ, Davison AM. Effect of renal replacement therapy on patients with combined acute renal and fulminant hepatic failure. Kidney Int Suppl. 1993;41:S245-S251. [PubMed] |
| 9. | Sato S, Suzuki K, Takikawa Y, Endo R, Omata M. Clinical epidemiology of fulminant hepatitis in Japan before the substantial introduction of liver transplantation: an analysis of 1309 cases in a 15-year national survey. Hepatol Res. 2004;30:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Akdogan M, Camci C, Gurakar A, Gilcher R, Alamian S, Wright H, Nour B, Sebastian A. The effect of total plasma exchange on fulminant hepatic failure. J Clin Apher. 2006;21:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Sekido H, Matsuo K, Takeda K, Ueda M, Morioka D, Kubota T, Tanaka K, Endo I, Togo S, Shimada H. Usefulness of artificial liver support for pretransplant patients with fulminant hepatic failure. Transplant Proc. 2004;36:2355-2356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Matsui A, Mochida S, Ohno A, Nagoshi S, Hirose T, Fujiwara K. Plasma osteopontin levels in patients with fulminant hepatitis. Hepatol Res. 2004;29:202-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Hiraoka A, Horiike N, Akbar SM, Michitaka K, Matsuyama T, Onji M. Soluble CD163 in patients with liver diseases: very high levels of soluble CD163 in patients with fulminant hepatic failure. J Gastroenterol. 2005;40:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Mita A, Hashikura Y, Tagawa Y, Nakayama J, Kawakubo M, Miyagawa S. Expression of Fas ligand by hepatic macrophages in patients with fulminant hepatic failure. Am J Gastroenterol. 2005;100:2551-2559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Iwaki T, Sugimura M, Nishihira J, Matsuura T, Kobayashi T, Kanayama N. Recombinant adenovirus vector bearing antisense macrophage migration inhibitory factor cDNA prevents acute lipopolysaccharide-induced liver failure in mice. Lab Invest. 2003;83:561-570. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Balow JE, Rosenthal AS. Glucocorticoid suppression of macrophage migration inhibitory factor. J Exp Med. 1973;137:1031-1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 161] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Grasso RJ, Klein TW, Benjamin WR. Inhibition of yeast phagocytosis and cell spreading by glucocorticoids in cultures of resident murine peritoneal macrophages. J Immunopharmacol. 1981;3:171-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Ware AJ, Cuthbert JA, Shorey J, Gurian LE, Eigenbrodt EH, Combes B. A prospective trial of steroid therapy in severe viral hepatitis. The prognostic significance of bridging necrosis. Gastroenterology. 1981;80:219-224. [PubMed] |
| 19. | Theodossi A, Eddleston AL, Williams R. Controlled trial of methylprednisolone therapy in severe acute alcoholic hepatitis. Gut. 1982;23:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 84] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Rakela J, Mosley JW, Edwards VM, Govindarajan S, Alpert E. A double-blinded, randomized trial of hydrocortisone in acute hepatic failure. The Acute Hepatic Failure Study Group. Dig Dis Sci. 1991;36:1223-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 73] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Misra NC, Jaiswal MS, Singh RV, Das B. Intrahepatic arterial infusion of combination of mitomycin-C and 5-fluorouracil in treatment of primary and metastatic liver carcinoma. Cancer. 1977;39:1425-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Tanioka H, Tsuji A, Morita S, Horimi T, Takamatsu M, Shirasaka T, Mizushima T, Ochi K, Kiura K, Tanimoto M. Combination chemotherapy with continuous 5-fluorouracil and low-dose cisplatin infusion for advanced hepatocellular carcinoma. Anticancer Res. 2003;23:1891-1897. [PubMed] |