Published online Nov 7, 2006. doi: 10.3748/wjg.v12.i41.6634
Revised: July 12, 2006
Accepted: August 21, 2006
Published online: November 7, 2006
AIM: To evaluate survival in patients undergoing palliative resection versus non-resection surgery for primary colorectal cancer in a retrospective analysis.
METHODS: Demographics, TNM status, operating details and survival were reviewed for 67 patients undergoing surgery for incurable colorectal cancer. Palliative resection of the primary tumor was performed in 46 cases in contrast to 21 patients with non-resection of the primary tumor and bypass surgery. Risk factors for postoperative mortality and poor survival were analyzed with univariate and multivariate analyses.
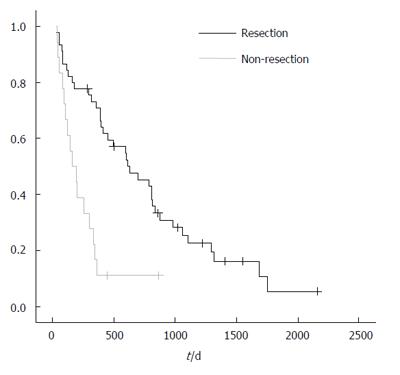
RESULTS: The two groups were comparable in terms of age, gender, preoperative presence of ileus and tumor stage. Multivariate analysis showed that median survival was significantly higher in patients with palliative resection surgery (544 vs 233 d). Differentiation of the tumor and tumor size were additional independent factors that were associated with a significantly poorer survival rate.
CONCLUSION: Palliative resection surgery for primary colorectal cancer is associated with a higher median survival rate. Also, the presence of liver metastasis and tumor size are associated with poor survival. Therefore, resection of the primary tumor should be considered in patients with non-curable colon cancer.
- Citation: Beham A, Rentsch M, Püllmann K, Mantouvalou L, Spatz H, Schlitt H, Obed A. Survival benefit in patients after palliative resection vs non-resection colon cancer surgery. World J Gastroenterol 2006; 12(41): 6634-6638
- URL: https://www.wjgnet.com/1007-9327/full/v12/i41/6634.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i41.6634
Colorectal cancer is the second leading cause of death by malignancy in western countries. About 30% of patients, detected by screening programs for colorectal cancer, present with distant metastases[1] and non-resectable distant metastases are the threshold for curative approaches in these patients. Regardless of the palliate situation, these patients often require surgical treatment due to acute complications such as bowel obstruction and perforation. In addition colon cancer can lead to chronic symptoms such as anaemia. In principal, two surgical approaches are optional. First, the primary colon cancer can be resected and the passage of the gut can be achieved by direct anastomosis or alternatively by a stoma. Second, the primary tumor can be left in place and the passage of the gut is achieved by bypassing the tumor or a stoma. Choices for surgical approach are based on several aspects. Frequently, patients with advanced colon cancer are poor risk candidates for surgery, considering that many of the patients are elderly with concomitant medical diseases and have been debilitated by the advanced malignancy. Obviously, leaving the primary tumor in place is often less demanding for the patient and surgical palliation can be as minimal as possible.
On the other hand, experimental evidence suggests that the primary tumor might control dormancy of distant metastasis. The phenomenon by which a primary tumor is able to inhibit growth of distantly spread foci of the same tumor has been demonstrated[2]. Thus removing the primary tumor in an advanced stage of colon cancer might promote metastatic progression and determine survival time of the patient.
We reviewed the results of non-curative surgery for patients with primary colorectal cancer and analyzed factors that might influence the operative mortality rate, postoperative morbidity and the survival of these patients.
Palliative surgery was defined as surgery with the presence of residual local disease in the operative field and the presence of non-resectable distant metastases. Between April 1993 and December 2003, 69 patients (45 men, 24 women; mean age 62 years, range 26-85) fulfilled study criteria and were subsequently entered prospectively into a database of colorectal malignancies in the Department of Surgery, University of Regensburg. Survival data, medical record, the locations of primary cancers and metastases, operative details, postoperative outcomes, and patients’ demographics were reviewed. Morbidity was defined as any post-operative complication that led to a prolonged hospital stay, additional procedures or post-operative mortality. Operative mortality was defined as death that occurred within 30 d from surgery. Two patients left Regensburg shortly after the surgery. Their follow-up data were incomplete, and they were excluded from the survival analysis.
Forty-five (65%) patients were admitted to the hospital with symptoms. The leading cause of admission was rectal bleeding in 39 (57%) patients. Urgent presentation with intestinal obstruction or perforation occurred in 5 patients. In addition to symptomatic patients, 21 (30%) of the tumors were detected by screening programs. Concomitant medical diseases were present in 47%, particularly prevalent in elderly patients (Table 1).
| Resection | Non-resection | Total | |
| Screnning Program | 16 (34) | 5 (23) | 21 (31) |
| Symptomatic | 28 (61) | 13 (61) | 41 (61) |
| Symptomatic with obstruction | 2 (4) | 3 (14) | 5 (7) |
Table 2 presents the sites of the primary cancer. Tumors distal to the splenic flexure were found in 54 (80%) patients. The TNM characteristics are listed in Table 3. No statistical difference between the two groups was found. The type of surgery is shown in Table 4. Resection of the cancer was performed in 46 of the 69 patients.
| Resection | Non-resection | Total | |
| Right colon | 13 (27) | 1 (5) | 14 (19) |
| Colon transversum | 1 (2) | 0 (0) | 1 (1) |
| Left colon | 2 (6) | 0 (0) | 2 (4) |
| Sigma | 16 (34) | 4 (19) | 20 (28) |
| Rectum | 14 (30) | 16 (76) | 30 (43) |
| Resection (n = 46) | Non-resection (n = 21) | Total (n = 67) | |
| cTx | 0 (0) | 4 (19) | 4 (6) |
| cT2 | 4 (9) | 0 (0) | 4 (6) |
| cT3 | 14 (32) | 5 (24) | 19 (29) |
| cT4 | 28 (60) | 12 (57) | 40 (59) |
| cN0 | 7 (15) | 2 (10) | 9 (16) |
| cN1 | 12 (26) | 6 (29) | 18 (26) |
| cN2 | 19 (41) | 0 (0) | 19 (28) |
| cN3 | 8 (16) | 0 (0) | 8 (11) |
| cN+ | 0 (0) | 13 (62) | 13 (19) |
| cM0 | 8 (17) | 8 (39) | 16 (24) |
| cM1 | 38 (82) | 13 (61) | 51 (75) |
| Resection | Non-resection | |
| Rectum resection | 13 (28) | |
| Rectum exstirpation | 3 (7) | |
| Sigma resection | 10 (28) | |
| Hemicolectomy left | 2 (4) | |
| Hemicolectomy right | 11 (24) | |
| Sigma + Rectum resection | 5 (11) | |
| Other Resections | 2 (4) | |
| Ileostomy | 21 (100) |
Categorical variables were analysed by the χ2 test or Fisher's exact test when appropriate. Continuous variables were presented in median values with range; they were analysed by the Mann-Whitney U test. Survival was calculated from the time of surgery. Analysis of survival excluded patients who died during the postoperative period. Survival data were analysed by the Kaplan-Meier method. Factors were compared by log-rank test. Multivariate analysis used the Cox proportional hazard model, and factors with P values less than 0.05 in univariate analysis were included in the multivariate analysis. Presentation of results includes hazard ratio (HR) and 95% confidence interval (CI). P values less than 0.05 were considered statistically significant.
Patients who underwent resection of the primary tumor or a non-resection procedure were comparable in terms of age, peritoneal seedings and operative mortality, as shown in Table 5. Furthermore, there was no difference in the presence of co-morbidities. Surgery was elective in 65 patients, and the remaining 2 were operated on as urgent surgery. The operative mortality rate was 4 patients, 3% with resection and 0% with non-resection procedure. Mean hospital stay was 15, 9 d (range 7-60). The median range of hospital stay (d) was not different between the two groups.
| Resection (n = 46) | Non-resection (n = 21) | |
| Sex: M/F | 29/17 (63/37) | 14/7 (67/33) |
| Median age | 61.6 | 63.7 |
| Peritoneal seedings | 7 (15) | 5 (24) |
| Operative mortality | 2 (4) | 0 (0) |
| Morbidity | 3 (7) | 0 (0) |
| Presence of distant metastasis | 38 (82) | 13 (62) |
| Median day of hospital stay (range) (postoperative mortality excluded) | 16.6 (7-60) | 14.7 (7-41) |
Post-operative therapy (47 chemotherapy alone, 2 radiation therapy, 6 combined chemoradiation) was given to 55 patients and was most prevalent for those who were younger in age or had a lower incidence of co-morbid medical diseases (Table 6). Since type of chemotherapy might influence the post-operative survival rate, stratification of the survival rates was performed according to the different drugs used. 12 different chemotherapeutic agents or combination of agents were used and no significant impact on survival was observed.
| Resection (n = 46) | Non-resection (n = 21) | |
| Chemo | 39 (85) | 8 (38) |
| Radiation | 0 (0) | 2 (10) |
| Chemo and radiation | 2 (4) | 4 (19) |
The median survival of patients with resection and without resection was 544 vs 233 d, respectively (P < 0.001; Figure 1).The analysis of risk factors that might affect the survival is shown in Table 7. Multivariate analysis showed poor survival to be associated with non-resection surgery (P < 0.001) and differentiation of the tumor (P < 0.001;
| Resection | Non-resection | |||
| n | median survival (d) | n | median survival (d) | |
| Sex | ||||
| Male | 29 | 742.1 | 14 | 218.7 |
| Female | 17 | 589.0 | 7 | 261.1 |
| Peritoneal metastasis | ||||
| Yes | 7 | 683.4 | 5 | 201.6 |
| No | 39 | 685.0 | 16 | 333.0 |
| Bilobar liver metastasis | ||||
| Yes | 38 | 599.7 | 13 | 220.4 |
| No | 8 | 578.2 | 8 | 253.1 |
| Resection | ||||
| Yes | 46 | 543.8 | 0 | |
| No | 0 | 21 | 232.9 | |
| Lymph nodea | ||||
| Positive | 37 | 660.0 | 19 | 225.8 |
| Negative | 9 | 790.8 | 2 | 300.0 |
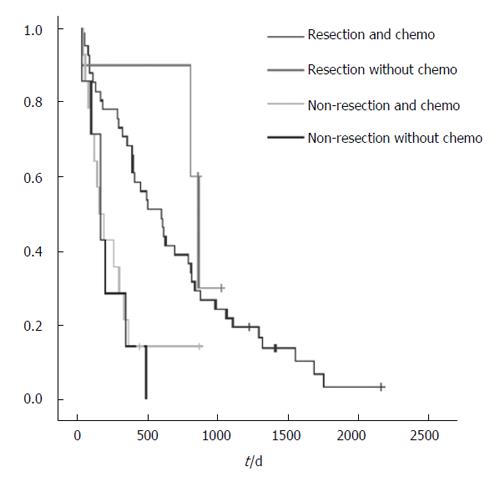
Patients with incurable colorectal cancer are usually compromised by the presence of advanced malignancy and many of them also suffer from concomitant medical diseases, rendering surgical treatment an option with high risk. Therefore, the main objectives of the operative treatment are avoiding complications such as obstruction, perforation and profuse bleeding. In addition, surgery is worthwhile only if a reasonable length of survival can be achieved and the operative risk is acceptable. At present, only a few studies with detailed analysis of the results of palliative surgery for primary colorectal cancer are available making surgical treatment for patients with incurable colorectal cancer controversial[3-6]. We evaluated survival in patients undergoing palliative resection versus non-resection surgery for primary colorectal cancer in a retrospective analysis and found a significant longer survival rate for patients with resected primary tumors. Interestingly, patients with resection of the primary tumor did not benefit from chemotherapy to a greater extent compared to non-resected patients.
Bleeding and obstruction are reported to be the main presenting symptoms in patients with palliative surgery[3,7] in colorectal cancer. 67% of our patients had symptoms of bleeding or obstruction. The symptoms were usually discrete resulting in only 3% of emergency operations. As an alternative to urgent surgery, self-expanding metallic stents have been discussed as an effective non-operative means to relieve acute colonic obstruction[8-12]. However, stents may dislocate or cause bleeding in up to 30% of stent-treated patients. Furthermore after dilatation of the obstruction with a stent the tumor is obviously left in place. None of our patients was treated with a stent because the main symptom was bleeding, but further development of this technique might reduce side effects and it may be a better alternative to surgical treatment.
Our approach to palliative surgery was aggressive, aiming for resection of the primary tumor. Non-resection was carried out in patients who were in very poor condition or if the resection was hazardous. This point of view has also been offered by other authors[4] and is seen as better palliation. The resection rate in our cohort was 66%, which is comparable to other studies ranging from 69%[5] to 90%[3]. In contrast to other reports, age or location (rectum or rectosigmoid) of the cancer did not influence the likelihood for resection. There was a tendency to perform non-resection in patients with peritoneal seeding.
The operative mortality in this study was 2.8%. This is lower than in other studies reporting non-curative surgery[3,5]. Both patients died after anastomosis leakage and subsequent peritonitis. Anastomosis in palliative surgery for rectal cancer has been advocated by others[13] reporting one case of anastomotic leakage in 60 patients with palliative anterior resection. Our rate of anastomosis leakage is comparable and thus we would not recommend avoiding anastomosis in a palliative situation. Restoring bowel continuity is not a hazardous procedure and can improve quality of life. Non-resection was not an independent factor for high post-operative mortality (none of the non-resected patients died post-operatively). This stands in contrast to other studies, which reported high mortality rates up to 17% in patients without resection, while rates in those with resection were 9% and 5%. However, it should be emphasized that in those studies the patients who did not receive resection had advanced diseases, severe co-morbidities or unstable haemodynamic status during surgery. Both groups of our patients were comparable in these respects and lower operative trauma might be reflected in the lower mortality of non-resected patients.
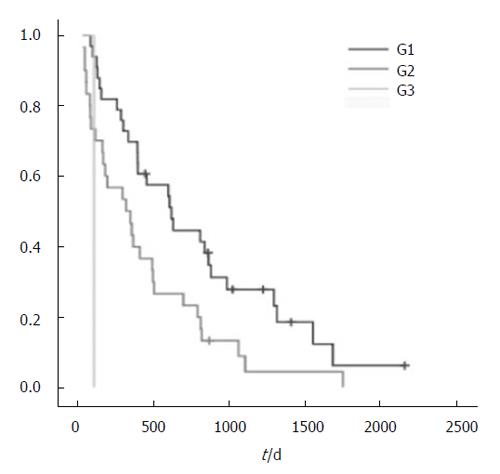
Others found that extensive liver involvement and poorly differentiated tumors, the presence of ascites and the absence of other therapies, age over 75 years, and cardiovascular disease were associated with poor survival. We have found that poorly differentiated tumors are associated with poor survival in surgery for incurable colorectal cancer in our patients. Only 5 patients were older than 75 years and therefore no relation between age and outcome could be demonstrated in our study. Others propose that surgical treatment is only worthwhile if the patients do not have severe symptoms. Obviously the decision may need individual consideration, and the potential benefits and risks should be balanced carefully.
Thus non-resection, which is associated with similar morbidity and mortality rates and hospital stay lengths as resection, may be the only option to palliate complications such as obstruction or perforation. The aim must be to discharge the patient to enjoy a mediocre quality of life in the remaining days. This option, however, must be weighted against overall survival. In our cohort, the resected patients had a significant benefit in survival. Thus the enhanced operative mortality versus better survival should be considered in the individual patient, and good clinical judgment is needed to balance the benefits and risks of surgery.
We also found that the use of therapeutic chemo-therapy and/or radiation therapy was not associated with better survival. Unfortunately, there were 12 different schemes of therapy given and thus no statistical difference was determined. There were only a few patients who did not receive palliative chemotherapy and thus no statistically significant level was reached. However, improved survival is likely to be achieved in current chemotherapeutic regimens. Traditional 5-fluorouracil based chemotherapy for metastatic colorectal cancer has a response rate of about 30%[14-18]. Better quality of life and improved survival have been demonstrated in patients who underwent chemotherapy than in those with the best supportive treatment[16]. Furthermore, irinotecan and oxaliplatin have been shown to produce a survival benefit and to improve quality of life in patients not responding to 5-fluorouracil based chemotherapy[19].
Taken together, palliative surgery for colorectal cancer is associated with an acceptable mortality rate, especially in patients without resection. Furthermore, anastomosis in a palliative situation is a safe procedure enhancing the quality of live for the patient. In addition, leaving the primary tumor in place is associated with poor survival. In the presence of these poor risk factors, good clinical judgment and careful consideration of balance between the risks and benefits are necessary before embarking on surgical palliation.
S- Editor Wang J L- Editor Lutze M E- Editor Ma WH
| 1. | Devesa JM, Morales V, Enriquez JM, Nuno J, Camunas J, Hernandez MJ, Avila C. Colorectal cancer. The bases for a comprehensive follow-up. Dis Colon Rectum. 1988;31:636-652. [RCA] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Guba M, Bosserhoff AK, Steinbauer M, Abels C, Anthuber M, Buettner R, Jauch KW. Overexpression of melanoma inhibitory activity (MIA) enhances extravasation and metastasis of A-mel 3 melanoma cells in vivo. Br J Cancer. 2000;83:1216-1222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Liu SK, Church JM, Lavery IC, Fazio VW. Operation in patients with incurable colon cancer--is it worthwhile. Dis Colon Rectum. 1997;40:11-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Rosen SA, Buell JF, Yoshida A, Kazsuba S, Hurst R, Michelassi F, Millis JM, Posner MC. Initial presentation with stage IV colorectal cancer: how aggressive should we be. Arch Surg. 2000;135:530-534; discussion 534-535;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 189] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Makela J, Haukipuro K, Laitinen S, Kairaluoma MI. Palliative operations for colorectal cancer. Dis Colon Rectum. 1990;33:846-850. [RCA] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Ruo L, Gougoutas C, Paty PB, Guillem JG, Cohen AM, Wong WD. Elective bowel resection for incurable stage IV colorectal cancer: prognostic variables for asymptomatic patients. J Am Coll Surg. 2003;196:722-728. [RCA] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 213] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Joffe J, Gordon PH. Palliative resection for colorectal carcinoma. Dis Colon Rectum. 1981;24:355-360. [RCA] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Law WL, Choi HK, Lee YM, Chu KW. Palliation for advanced malignant colorectal obstruction by self-expanding metallic stents: prospective evaluation of outcomes. Dis Colon Rectum. 2004;47:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | De Gregorio MA, Mainar A, Tejero E, Alfonso E, Gimeno MJ, Herrera M. Use of an introducer sheath for colonic stent placement. Eur Radiol. 2002;12:2250-2252. [PubMed] |
| 10. | Mainar A, De Gregorio Ariza MA, Tejero E, Tobío R, Alfonso E, Pinto I, Herrera M, Fernández JA. Acute colorectal obstruction: treatment with self-expandable metallic stents before scheduled surgery--results of a multicenter study. Radiology. 1999;210:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 130] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Tejero E, Fernandez-Lobato R, Mainar A, Montes C, Pinto I, Fernandez L, Jorge E, Lozano R. Initial results of a new procedure for treatment of malignant obstruction of the left colon. Dis Colon Rectum. 1997;40:432-436. [RCA] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Tejero E, Mainar A, Fernandez L, Tieso A, Cuezva JF, San Jose A. New procedure for relief of malignant obstruction of the left colon. Br J Surg. 1995;82:34-35. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Moran MR, Rothenberger DA, Lahr CJ, Buls JG, Goldberg SM. Palliation for rectal cancer. Resection Anastomosis. Arch Surg. 1987;122:640-643. [RCA] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Petrelli N, Herrera L, Rustum Y, Burke P, Creaven P, Stulc J, Emrich LJ, Mittelman A. A prospective randomized trial of 5-fluorouracil versus 5-fluorouracil and high-dose leucovorin versus 5-fluorouracil and methotrexate in previously untreated patients with advanced colorectal carcinoma. J Clin Oncol. 1987;5:1559-1565. [PubMed] |
| 15. | Doroshow JH, Multhauf P, Leong L, Margolin K, Litchfield T, Akman S, Carr B, Bertrand M, Goldberg D, Blayney D. Prospective randomized comparison of fluorouracil versus fluorouracil and high-dose continuous infusion leucovorin calcium for the treatment of advanced measurable colorectal cancer in patients previously unexposed to chemotherapy. J Clin Oncol. 1990;8:491-501. [PubMed] |
| 16. | Scheithauer W, Rosen H, Kornek GV, Sebesta C, Depisch D. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ. 1993;306:752-755. [RCA] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 464] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 17. | Cazacu M, Oniu T, Lungoci C, Mihailov A, Cipak A, Klinger R, Weiss T, Zarkovic N. The influence of isorel on the advanced colorectal cancer. Cancer Biother Radiopharm. 2003;18:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Baars A, Buter J, Pinedo HM. Making use of the primary tumour. Bioessays. 2003;25:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Cunningham D, Pyrhonen S, James RD, Punt CJ, Hickish TF, Heikkila R, Johannesen TB, Starkhammar H, Topham CA, Awad L. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet. 1998;352:1413-1418. [RCA] [DOI] [Full Text] [Cited by in Crossref: 946] [Cited by in RCA: 888] [Article Influence: 32.9] [Reference Citation Analysis (0)] |