INTRODUCTION
Focal nodular hyperplasia (FNH) is a relatively rare benign liver tumor, often asymptomatic and discovered incidentally[1,2]. It occurs in both men and women, but shows a predilection for young women. FNH presents as a solitary lesion in 70% of the cases, while in 30% of patients two to five lesions are present[3]. Multiple lesions occur rarely[4-7].
Although an association with the use of oral contraceptives has been shown[6,7], its pathogenesis is still unclear. The potential for malignant transformation of FNH into hepatocellular carcinoma (HCC) has not been demonstrated[8]. However, cases of anatomical adjacency of fibrolamellar carcinoma (FL-HCC) and FNH in the same patient have been described, and some authors have suggested a direct link between the two tumors[9,10].
The simultaneous presence of HCC in its typical form and FNH is exceptional. To the best of our knowledge, only few cases regarding simultaneous occurrence of FNH and HCC of the liver have been reported in literature[8,11]. In this case report we describe a case of co-existent FNH and HCC and discuss the clinical management and therapeutic implications.
CASE REPORT
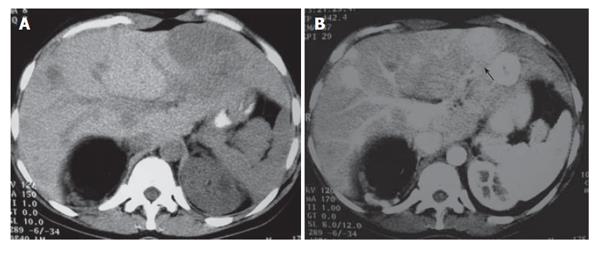
A 23-year-old woman was referred to the Outpatient Department of our hospital with a present history of fatigue and a past medical history of atresia of oesophagus which was reconstructed during her neonatal age and underwent oesophageal dilatations in her adolescence. She has never used oral contraceptives and had no history of hepatitis or alcohol abuse. Physical examination did not reveal any abnormal findings. Laboratory findings including serum α-fetoprotein and carcinoembryogenic antigen levels were within normal ranges. Contrast-enhanced abdominal CT scan showed five lesions in the liver (both left and right lobes), with the largest of the lesions located in the left lobe, an angiomyolipoma in the right kidney and multiple small angiomyolipomas of the left kidney. All liver lesions enhanced greatly during the early arterial phase (Figure 1A and B).
Figure 1 A: Unenhanced transverse CT scan demonstrates multiple hypoattenuating masses located on both liver lobes.
Fatty tissue (known angiomyolipoma) replaces the most of the upper pole of the right kidney; B: Post-contrast CT scan depicts multiple round liver lesions, with a smooth margin, which demonstrate intense homogeneous enhancement. The lesion located in segment three, has a small central area of hypodensity, consistent with a central scar.
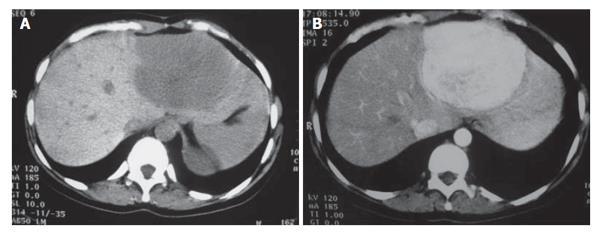
Three months later, a new contrast-enhanced abdominal CT scan was repeated and revealed apart from the known masses an increase in the size of the tumor in the left lobe of the liver (diameter from 4 cm to 7 cm). On unenhanced scans all masses appeared well-defined and homogeneously hypodense. Following an i.v. bolus of contrast, the masses showed early arterial contrast enhancement, with the exception of a centrally located area in the largest lesion, which remained hypodense (Figure 2A and B). On portal venous phase scans all lesions became isodense. The central hypodense area of the largest lesion remained unchanged during the portal venous phase scans, while became hyperdense on delayed scans. This area was attributed to a central scar, a finding consistent with a typical FNH. The remaining three masses were also considered as FNHs. MR imaging was carried out at the same time and the findings were consistent with multiple FNHs.
Figure 2 A: Pre-contrast CT scan 3 mo later, revealing the significant increase in the size of the lesion located in the left liver lobe; B: Post-contrast image at the same level showing the increased contrast uptake of the lesion.
Due to the great increase in the size of the mass located in the left lobe, during such a short period (3 mo), a CT guided core liver biopsy was performed in the largest lesion. Pathologic examination of the biopsy revealed that the specimen composed of fibrous tissue surrounded a nodule of hyperplastic hepatocytes, contained numerous thin-walled vessels, as well as numerous proliferated bile ductules. No evidence of malignancy was observed. Based on the radiological and histopathological data, the provisional diagnosis of FNH was made. In order to exclude any coexistent brain pathology, the patient underwent brain MR imaging, which was normal.
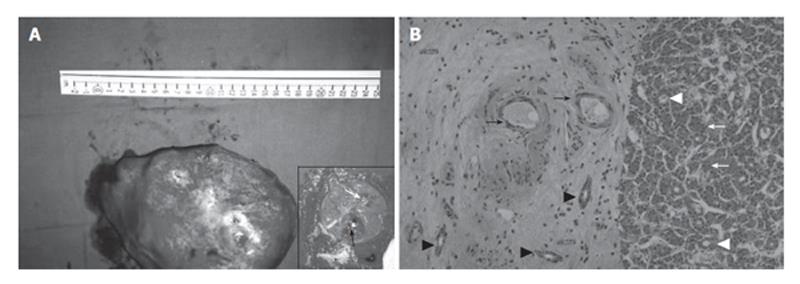
The surgical procedure involved the resection of the II and III liver segments. During the operation a frozen section was performed, revealing FNH. The surgical specimen measured 17.3 cm × 15.0 cm × 10.2 cm (Figure 3A). It consisted of a portion of liver which contained two masses, the larger measuring 9 cm × 6 cm × 5 cm and the smaller 4.5 cm × 3.5 cm × 2.0 cm. Macroscopically, both masses had a yellow-white cut surface and a central scar. In addition, the smaller mass contained another smaller tumor measuring 2.1 cm × 1.8 cm × 1.0 cm, which was located in the periphery and showed a brown-green cut surface (Figure 3A-inset). Microscopic examination of the specimen revealed the presence of FNH (two discrete tumors), whereas the smaller tumor included within the smaller mass represented a well-differentiated hepatocellular carcinoma (Figure 3B). Diagnosis of HCC was based on morphological (HE stain) and immunohistochemical grounds (Figure 4): The neoplastic cells exhibited positive stain for hepatocyte paraffin antigen and cytokeratin 18 and negative stain for cytokeratin CK19, chromogranin, synaptophysin, common leukocyte antigen negative, CD34 antigen. In addition, polyclonal carcinoembryonic antigen displayed a canalicular pattern.
Figure 3 A: Photograph of the surgical specimen.
The inset shows the cut surface: White arrow points at the scar of FNH and black arrow shows the hepatocellular carcinoma; B: Photomicrograph from the lesion. Left: Connective tissue from the core of FNH containing thin wall vessels (black arrows) and cholangioles (black arrowheads). Right: hepatocellular carcinoma. The tumor shows a trabecular growth pattern (white arrows) and focal pseudoglandular transformation (white arrowheads) (HE x 100).
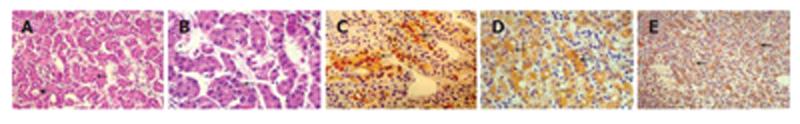
Figure 4 Microphotograph showing details of the tumor.
A, B: The tumor displays a trabecular growth pattern (A-arrow, B-arrow) with focal pseudoglandular transformation (A-arrowhead) (HE, A × 250, B × 400); C: Tumor cells show positive immunohistochemical stain for hepatocyte antigen (arrows-streptavidin biotin peroxidase × 250); D: Tumor cells show positive immunohistochemical stain for cytokeratin 18 (arrows-streptavidin biotin peroxidase × 400); E: Immunohistochemical stain for polyclonal carcinoembryonic antigen displays a canalicular pattern (arrows-streptavidin biotin peroxidase × 100).
The postoperative period was uneventful. During the follow-up period, the patient underwent liver transplantation in another center abroad. Pathologic examination at that time revealed that the two small tumors in the right liver lobe represented liver angiomyolipomas (personal communication). At present time, eight years after the initial diagnosis, the patient is alive.
DISCUSSION
Benign liver tumors are uncommon in surgical practice (3%-5% of all liver tumors); haemangioma is the most common type (55%-60%), whereas adenomas (8%-19%) and FNH (21%-27%) are less frequent. FNH, also called mixed tumor represents a small percentage (1%-5%); its definition is derived from the coexistence of two or more tumor phenotypes[1,12,13]. FNH is generally considered to be a hyperplastic response to an abnormal blood supply[14].
The potential of benign liver tumors for malignant degeneration has been extensively discussed in the literature; in particular, a considerable risk of malignant transformation has been reported for hepatic adenoma[15], which was supported by findings of HCC foci within the benign tumor mass. This is why surgical resection of hepatic adenomas is recommended by most authors (apart from considerations about possible rupture of larger adenomas and subsequent intra-abdominal bleeding).
Conversely, there is no agreement on the malignant potential of FNH. To the best of our knowledge only a few cases of co-existent FNH and HCC have been reported in the literature[8,11] and the pathological correlation is not always clear. Saul et al reported one case in 1987 in which FNH and HCC were concomitant, although the HCC was a fibrolamellar variant (FL-HCC)[9]; Chen et al recently reported one case of HCC partially surrounded by FNH[8], while even more recently Cucchetti et al reported a case of simultaneous presence of FNH and HCC[11].
Simultaneous appearance of FNH with adenoma[16,17] and haemangioma[18] has been reported. The simultaneous occurrence of adenoma, focal nodular hyperplasia and haemangioma has also been described and the authors have concluded that adenoma, FNH and haemangiomas might have a common origin that should be attributed to vascular changes primarily caused by a congenital abnormality of the angioarchitecture and blood circulation of the liver[19]. Various authors have described the co-existence of FNH with vascular cranial malformations[20], cystic dysplasia of the kidneys[21], Klippel-Trenaunay syndrome[22]. In all cases described so far, the coexistence of the above findings were attributed to a so called syndrome or were described as coincidental[23]. A useful diagnostic tool for the distinction between liver cell adenoma and FNH, when the routine histopathologic features are not very clear, is clonality analysis[24].
The histopathological features of FL-HCC suggest a direct link between this tumor and FNH and some authors hypothesize a direct evolution from FNH to FL-HCC[9,10]. Also, the simultaneous presence of adjacent adenoma does not exclude the development of HCC from malignant degeneration of the adenoma. Therefore co-existence of FNH and HCC in the same patient is an exceptional event, to the best of our knowledge reported only in two cases.
Recent insight into the molecular characteristics of the clonal growth of FNH failed to support further a possible derivation of HCC from FNH; two different studies, conducted by Gaffey[12] and Paradis[13], were undertaken to clarify the monoclonal or polyclonal nature of these tumors by a method that scans the molecular pattern of inactivation of chromosome X. The authors eventually came to opposite conclusions. In another study[8], clonal analysis was applied to macroscopically different portions having different histological features within the same tumor, with concomitant FNH and HCC; the results showed that FNH was of monoclonal origin, but the FNH clone was similar to that of HCC and therefore the authors’ conclusions did not support the hypothesis that HCC was the product of malignant transformation from FNH. The issue of an identical clonal origin of FNH and HCC therefore remains a matter of debate, although we feel it is rather imprudent at present to completely exclude any malignant potential of FNH.
The potential of FNH for malignant evolution would appear unlikely on the basis of the follow-up of patients with non-surgically excised FNH. Weimann et al observed an increase in the size of FNH lesions only in 9.5% of 53 cases, with no malignant transformation during a mean follow-up of three years[25]. Likewise, in other studies no increase in lesion size was observed among 11 patients in a two-year follow-up, and in some cases the FNH even completely disappeared over time[26]. This evidence recently led Charny et al, to conclude that, if the diagnosis is unequivocal, surgical resection is not indicated for asymptomatic FNH[27].
A clear and precise diagnosis of a benign liver tumor is difficult to obtain in every patient, particularly in the case of FNH. In the series collected by Terkivatan, difficulty in differentiating FNH from adenoma or HCC represented the indication for surgery in 32% of cases[28]. FNH was rarely encountered by radiologists prior to the current practice of helical multiphasic CT or MR imaging. It is also believed that as the imaging methods improve, FNH will be encountered more frequently. Many authors have reported the CT features of FNH. Imaging characteristics, typical of FNH, include a homogeneous well-defined hypo-, or iso-dense lesion on unenhanced scans, which shows bright enhancement during early arterial phase and becomes isodense on portal venous phase. Central scars are hypodense on early arterial and portal venous phases and become hyperdense on delayed scans[29-32]. Magnetic resonance imaging is another modality also used for the confident diagnosis of FNH. The reported sensitivity and specificity values for contrast enhanced-MRI diagnosis of FNH was 70% and 98% respectively in an article by Cherqui et al in a series of 41 patients with clinical radiological and pathological correlations. The central scar was detected in 78% of the cases[33]. An alternative to angiography could be hepatic cholescintigraphy, which according to the study of Weimann, best reflected the vascular pattern and the typical biliary ductule proliferation of FNH (sensitivity ≥ 82%, specificity ≥ 97%)[25].
Finally, the diagnostic value of liver biopsy in suspected FNH is rather limited; the lesion itself may not be reached or the specimen may not be sufficient for an accurate diagnosis, and false negative results are deleterious for the final outcome as hepatic adenomas or HCC may bleed or seed along the needle track.
In conclusion, at present there is no clear-cut evidence supporting the potential for malignant degeneration of FNH into HCC; the indication for surgery, particularly in small lesions (≤ 4 cm) and asymptomatic patients, is therefore rather controversial. In view of this uncertainty, a correct diagnosis which differentiates between FNH and HCC must be achieved for all cases by means of a multidisciplinary approach. Due to the rarity of the association between FNH and HCC it is difficult to draw solid conclusions for both the pathology of this entity and the appropriate management of these patients.