INTRODUCTION
Surgical resection of colorectal liver metastases is nowadays a standard of care for resectable disease with 5-year survival rate approaching 60%[1-3]. Because of several theoretical benefits, preoperative systemic chemotherapy has been frequently used to downsize the disease.
We report here the development of two complications, partial portal vein thrombosis and hepatic steatosis with lobular inflammation, during the course of preoperative chemotherapy with FOLFIRI plus bevacizumab for colorectal liver metastases, and discuss the surgical management and implications.
CASE REPORT
A 61-year-old woman was referred for treatment of synchronous multiple bilobar colorectal liver metastases. Six months after a laparoscopic right hemicolectomy for a pT3N2 colon cancer, a multiphase spiral computed tomography (CT) performed without and with intravenous (IV) contrast revealed bilateral disease with a total of five lesions distributed in segmentsI, II, IVA, VI and VII amenable to hepatic resection (Figure 1). Because of the known high risk of disease recurrence after liver resection for multiple bilobar and synchronous metastases in patients with N2 classification of primary tumor, the patient was treated with six courses of preoperative chemotherapy with FOLFIRI (irinotecan, 5-fluorouracil, and high-dose leucovorin) plus bevacizumab.
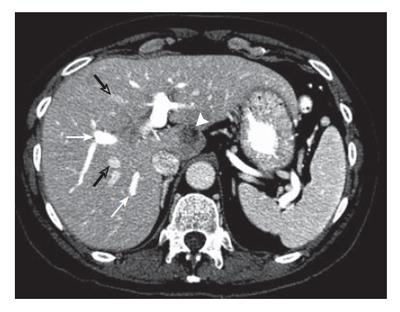
Figure 1 Contrast enhanced CT at the level of the portal bifurcation before treatment shows metastasis in segment I (arrowhead), opacified right anterior and posterior segmental portal veins (white arrows), and opacified middle and right hepatic veins (open arrows).
Repeat CT performed without and with IV contrast 3 wk after the completion of chemotherapy with bevacizumab showed downsizing of the lesions in segmentsI, IVA, VI-VII, and stable disease in the segment II. Non-contrast images also showed the development of diffuse fatty infiltration of the liver with sparing of segments V and VIII while the images acquired after IV contrast administration revealed absent portal perfusion of the right anterior sector associated with right anterior portal branch occlusion. The lack of portal flow to segments V and VIII explained sparing from steatosis and increased arterial flow to these segments. Some compensatory hypertrophy of the left liver was evident (Figure 2). The patient had no evidence of hypercoagulation and no other known risk factors for thrombosis. Even though this patient experienced downsizing of bilobar liver metastases, the new finding of portal vein thrombosis led to discontinuation of chemotherapy and reassessment of the treatment plan. Prior to occurrence of portal vein thrombosis, a partial right hepatectomy plus resection of the metastases in segments IVA, II andI, was planned. The finding of right portal vein thrombosis led to a change in operative plan to include resection of the segments involved both by tumor and by portal vein thrombosis in a 2-stage fashion. At the first stage, segmental resection of the lesions in segmentsIand II was performed, preserving the majority of the parenchyma of the lateral bisegment. Three-dimensional liver volumetry revealed the planned remnant liver (bisegment II/III) was of inadequate volume to permit resection of the right liver plus segment IV. Therefore, in order to increase volume and function of the liver remnant prior to the 2-stage resection, percutaneous right portal vein embolization extended to segment IV was performed. Repeat CT volumetry after 4 wk revealed adequate liver remnant hypertrophy, and the remaining diseased liver was resected by extended right hepatectomy (resection of Couinaud segments IV through VIII). The postoperative course after each stage was uneventful. The pathological review of the specimen revealed 50% necrosis in the tumors and moderate-severe steatosis with lobular inflammation in the resected liver.
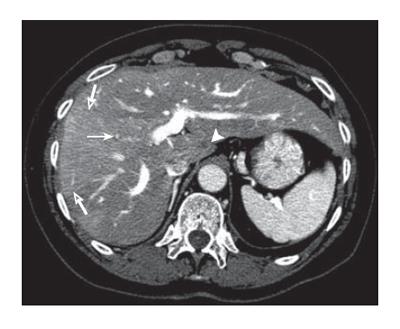
Figure 2 Contrast enhanced CT at the level of the portal bifurcation after treatment shows improved hepatic metastasis in segment I (arrowhead), non-visualization of the thrombosed anterior right segmental portal vein (arrow), opacified posterior segmental portal veins, middle and right hepatic veins, and wedge shaped increased enhancement (open arrows) of the anterior sector secondary to portal vein occlusion.
DISCUSSION
We report the development of partial portal vein thrombosis in combination with hepatic steatosis and lobular inflammation (steatohepatitis) during the course of preoperative chemotherapy with FOLFIRI plus bevacizumab for colorectal liver metastases. This thrombotic complication, which occurred during chemotherapy, was possibly not because of the presence or treatment effect of a metastatic lesion since no tumor was associated with the thrombosed portal vessel. Absence of previous data on vascular events in association with 5-fluorouracil alone, a drug which has been in use for more than 50 years, raises the possibility of an event associated with bevacizumab and/or irinotecan treatment.
Bevacizumab is a humanized recombinant murine monoclonal antibody to vascular endothelial growth factor A (VEGF-A) that competitively blocks binding of VEGF-A to its receptors, resulting in inhibition of angiogenesis. Bevacizumab has been reported to be associated with delayed wound healing, venous and arterial thromboembolism; however, to our knowledge bevacizumab has not been reported to cause portal venous thrombosis[4-8]. The proposed mechanism of bevacizumab-related thrombosis is complex-both hemorrhagic and thrombotic events may be involved. Briefly, bevacizumab by antagonizing VEGF’s functions might decrease the renewal capacity of endothelial cells in response to trauma, leading simultaneously to a tendency to bleeding and thrombosis[9,10].
Preliminary evidence that irinotecan may be associated with arterial and venous thrombotic events has been reported as well, but portal vein thrombosis has not been reported previously and the pathophysiology of irinotecan-related thrombotic events remains unclear[6,11].
The baseline incidence of thrombosis in cancer patients is significant, and the development of portal vein thrombosis in our patient cannot be definitively attributed to chemotherapy[12]. However, the pattern of thrombosis, the occurrence during chemotherapy treatment, and the absence of any vascular complications after colectomy, portal vein embolization or 2 sequential hepatectomy procedures, suggest that combination of FOLFIRI and bevacizumab contributed to this complication.
Histopathologic review of the resected liver confirmed not only moderate-severe steatosis consistent with the CT findings, but also revealed lobular inflammation[13]. Steatosis plus lobular inflammation can progress to steatohepatitis, which has been associated with preoperative chemotherapy with irinotecan, and may raise the risk of death after liver surgery[14-17]. Adverse outcomes related to chemotherapy-induced injury likely relate to a decreased regenerative capacity of injured hepatocytes in response to major hepatectomy, possibly through alterations of nuclear factors such as nuclear factor-kappa B, which is crucial for the priming phase of liver regeneration[18].
In the patient reported, who had resectable disease at presentation, development of both portal vein thrombosis and hepatic steatosis suggests that chemotherapy treatment contributed to these events and raised the possibility that curative surgery would not be possible. Fortunately, the recognition of these complications led to timely discontinuation of chemotherapy as well as a change in the surgical strategy to resect the tumors and the damaged liver through advanced techniques (portal vein embolization, extended hepatectomy, 2-stage approach) in an effort to minimize morbidity.
Chemotherapy will be used with more frequency before hepatic resection. Duration of treatment and drug doses and combinations may impact the development of chemotherapy-induced liver injury. Surgeons and medical oncologists must work together to devise safe, rational, and oncologically appropriate treatments for patients with multiple colorectal liver metastases, and to improve the understanding of the pathogenesis of chemotherapy-induced liver injury.