Published online Oct 28, 2006. doi: 10.3748/wjg.v12.i40.6536
Revised: May 12, 2006
Accepted: May 22, 2006
Published online: October 28, 2006
AIM: To investigate the role of the polymorphism of p53 codon 72 in early gastric cancer (EGC) and advanced gastric cancer (AGC) in Korean patients.
METHODS: DNA was extracted from blood samples of gastric cancer patients (n = 291) and controls (n = 216). In the p53 codon 72 genotypes were determined by PCR-RFLP.
RESULTS: Patients with gastric cancer had a significantly higher frequency of the homozygous proline (Pro) allele than the control (P = 0.032). Patients with AGC had a significantly higher frequency of the Arg/Arg (arginine) allele (P = 0.038) than EGC and a similar Pro/Pro allele. The signet ring cell type had a higher frequency of the Pro/Pro allele than other types (P = 0.031). The Pro/Pro genotype carries a 3.9-fold increased risk of developing gastric cancer (95% CI, 1.3-15.4, P = 0.039) when compared to Arg/Arg and Arg/Pro genotypes and to develop EGC is a 5.25 fold increased risk (95% CI, 1.8-19.6, P = 0.021).
CONCLUSION: The Pro/Pro genotype of the p53 codon 72 polymorphism carries a higher risk for gastric cancer in general and is also associated with a much higher risk for EGC than AGC.
-
Citation: Yi SY, Lee WJ. A
p53 genetic polymorphism of gastric cancer: Difference between early gastric cancer and advanced gastric cancer. World J Gastroenterol 2006; 12(40): 6536-6539 - URL: https://www.wjgnet.com/1007-9327/full/v12/i40/6536.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i40.6536
Gastric cancer is one of the most common malignancies worldwide, although the overall incidence of gastric cancer has been decreasing over the past few decades. Chronic H pylori infection and dietary factors, such as those high in salt or nitrate, and nutritional deficiencies have been associated with gastric cancer[1]. Gastric carcinogenesis is a complex, multistep, and multifactorial process, in which many factors are implicated. The majority of gastric cancers are thought to be caused by environmental factors that result in damage to the mucosa and that inhibit its ability to repair itself[2]. This response is regulated, in part, by inhibitory and stimulatory factors that are products of proto-oncogenes and tumor suppressor genes[3].
TP53 gene, an important tumor suppressor gene, encoded p53 protein. The p53 tumor suppressor protein was initially isolated in 1979 as a 53 kDa protein that was associated with SV 40 large T antigen[4]. It was a decade before p53 was recognized as an important tumor suppressor because of its frequent mutation in human cancers. A large number of human cancers show the evidence of inactivation of the p53 pathway, suggesting that malignant transformation requires reduction or elimination of p53’s function as “guardian of the genome”. It is estimated that up to 50% of human cancers carry a mutation of the p53 gene[5,6].
Germ line polymorphism of genes involved in multiple steps of carcinogenesis may also account for genetic difference in stomach cancer susceptibility. The p53 gene is the most intensively studied human gene because of its role as a central tumor suppressor, and has been widely studied in gastric cancer. However, although more than 75% of gastric cancer showed p53 overexpression, less than 30% had mutation in this gene[7]. The codon 72 polymorphism is located in exon 4 of the p53 gene, a region involving very few mutations[8]. At least two forms of wild-type p53 protein exist among major human populations; these forms are ascribed to amino acid replacement at codon 72 of Arg (CGC) by Pro (CCC) in the domain of transactivation of the p53 protein, though the functional difference between them is unknown. Pro variant allele of this p53 polymorphism has been studied as a potential risk factor for cancer of the lung, breast, and large bowel, with inconsistent results. Shepherd et al[8]. examined the relationship between codon 72 polymorphism and their susceptibilities to gastric cancer in a group of American gastric cancer patients.
In this study, we examined the genotypic frequency of codon 72 in early gastric cancer (EGC) and advanced gastric cancer (AGC) in 292 Korean patients to investigate the role of the p53 polymorphism.
Two hundred twenty two diagnosed gastric cancers were recruited from Ewha Womans University Mokdong Hospital from 2001 to 2005. Their mean age was 56 years (range, 26-88 years); 171 were males and 121 were females. Of the 292 patients, 189 (64.7%) showed advanced gastric cancer, 103 (35.3%) showed early gastric cancer. Two hundred sixteen controls were randomly selected from subjects attending routine medical check-ups (mean age 58 years; range 24-85 years; 128 males and 88 females) who were not affected with stomach cancer by endoscopy. Their details are presented in Table 1. Blood specimens, including serum, plasma, and white blood cells, from the study subjects were also obtained and frozen at -70°C for subsequent analysis. Informed written consent was obtained from all the enrolled patients.
| n | |
| Median age | 56 (22 -88) |
| Gender | |
| Male | 171 |
| Female | 121 |
| Type of stomach cancer | |
| Early cancer (EGC) | 103 |
| Advanced cancer (AGC) | 189 |
| Codon 72 polymorphism | |
| Arg/Arg | 101 |
| Arg/Pro | 126 |
| Pro/Pro | 65 |
| Laurence classification | |
| Intestinal type | 140 |
| Diffuse type | 152 |
| Differentiation | |
| Well/Moderate | 114 |
| Poor | 132 |
| Signet ring cell | 46 |
Genomic DNAs were isolated from peripheral whole blood, 200 μL (which has been treated with EDTA) by a DNA purifying kit (QIAamp DNA kit Blood Mini Kit, Qiagen, Germany, Hilden) according to the manufacture’s instructions.
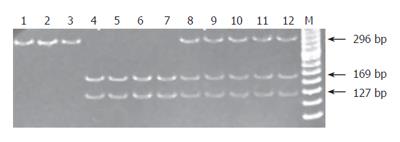
Genotyping of p53 at codon 72 in exon 4 [M22884. Human phosphoprotein (gi:189467)] was carried out by a polymerase chain reaction (PCR) amplification procedure using primers (p53-S: 5’-ATC TAC AGT CCC CCT TGC CG-3 and p53-AS: 5’-GCA ACT GAC CGT GCA AGT CA-3’). The amplification reaction was performed in a 6 μL (0.1 μg/μL) genomic DNA template, 0.1 μL (10 nmol/mL) of each primer, 1.6 μL (5 mmol/L per mL) dNTP, 0.1 μL (0.5 U/µL) Taq polymerase (Promega, Madison, WI, USA), and 2 μL of 10X reaction buffer (200 mmol/L per mL Tris-HCL (pH8.3), 500 mmol/L per mL KCL, and 30 mmol/L per mL MgCl2). PCR was carried out by 30 cycles under the following conditions: 1 min at 95°C for denaturation, 1 min at 62°C for primer annealing, and 1 min at 72°C for primer extension, using the GeneAmp PCR System 9600 (Applied Biosystem, Foster City, CA). The PCR product was visualized on a 2% agarose gel by electrophoresis, followed by ethidium bromide staining. This generates a 296-base pair fragment. The restriction enzyme BstUI (10 unit, New England Biolabs, Berverly, MA) digests (for 3 h at 60°C) within the sequence corresponding to the Arg codon (CGC) at position 72 to generate two visible fragments of 169 bp and 127 bp and leaves the Pro allele uncut (Figure 1).
Frequency tables were constructed using the SPSS (11.0 version) statistical package with statistical significance using the χ2 test. The odd ratios and 95% Confidence interval (CI) were calculated as an approximation of relative risk and adjusted for confounding factors such as age and gender using a logistic regression model.
We determined the frequency of the three phenotypes of the p53 gene in the patients with stomach cancer and controls (Table 2). Genotypes Arg/Arg, Arg/Pro, and Pro/Pro were found 41.2%, 47.7%, and 11.1% in individual controls and 34.5%, 43.1%, and 22.3% in the patients of stomach cancer, respectively. Distribution patterns of the germ line p53 polymorphism of the patients with stomach cancer included EGC and AGC showed in Table 2. We observed a dramatically increased frequency of Pro/Pro allelotype in stomach cancer patient, especially in the patients of EGC.
A logistic regression analysis suggests that the homozygous 72 Pro genotype carries a 3.9-fold increased risk of developing gastric cancer (95% CI, 1.3-15.4, P = 0.039) when compared to Arg homozygous and Arg/Pro heterozygous. The risk for 72 Pro homozygous patients to develop early gastric cancer is 5.35 (95% CI, 1.8-19.6, P = 0.021).
We examined the frequency difference in each genotype of p53 by histological type of all stomach cancers. Germ line p53 polymorphism was associated with stomach cancer, especially the signet ring cell type of adenocarcinoma (P = 0.031, Table 2). There was no relationship between patient gender, tumor stage, the depth of invasion in the wall, histologic type of cancer (Laurence classification: intestinal and diffuse types) and the distribution of codon 72 genotypes.
The identification of genes involved in cancer development is critical for uncovering the molecular basis of cancer. The p53 tumor suppressor protein is essential in the control of cell growth, apoptosis and the maintenance of genomic stability. Loss of p53 function caused by genomic alterations or interaction with environmental and bacterial products has been suggested as a critical step in multistage human carcinogenesis[9] .
The p53 gene consists of 11 exons; exons 2-11 code for the protein of 393 aminoacids. The majority of p53 mutations identified have been found in exons 5-8. However, mutations outside exons 5-8 may occur and they were chiefly observed in exons 4 and 10[10,11]. At least 10 different polymorphisms have been detected in the human genomic p53[12]. The functional significance of these polymorphisms is currently unknown. The hypothesized relationship between the codon 72 p53 polymorphism and cancer susceptibility dose not have any mechanistic basis. The Pro variant allele of the p53 polymorphism at codon 72 may not directly affect the p53 function. It may be in linkage disequilibrium with an as-yet-unidentified functional polymorphism. However, the single-codon difference of the p53 gene has been demonstrated to result in structurally different proteins[13]. The polymorphism is localized within a region of polypeptide that was lacking in a deletion mutant of mouse p53 that had an enhanced ability to immortalize primary rat cells[14,15].
In a literature review, there was little report about investigation of p53 gene polymorphism between AGC and EGC comparing with control. We have observed that TP53 codon 72 genotype in stomach cancer and control subjects. Our data further suggest that a p53 genetic polymorphism was associated with the susceptibility for stomach cancer, especially advanced stomach cancer.
There were several studies conducted to investigate the association between the codon 72 p53 polymorphism and lung cancer or gastric cancer. The risk increased approximately twofold for smoking-related lung cancer among individuals carrying the Pro/Pro genotype compared with those with other genotypes of the codon 72 p53 polymorphism[16-18]. A recent study in the patients of gastric cancer showed the significant difference from healthy control, with 48.6% Arg/Arg and 3.6% Pro/Pro in gastric cancer patients compared with 41.5% and 10.9% in healthy controls[19]. They showed an increased frequency of Arg/Arg genotype in cancer patients at age 75 or more than at a younger age. They suggested the prognosis in patients with Pro allele (proline homozygote or Pro/Arg heterozygotes) was worse than that those with Arg/Arg genotype. They also showed that preferential frequency of codon 72 Arg p53 acts as a survival factor in gastric cancer patients who have homozygous Arg alleles which confer a late start of gastric cancer when compared with those with the Pro allele.
In this study we found that the distribution of genotypes had significant difference between the patients of stomach cancer and controls, with 34.5% Arg/Arg and 22.3% Pro/Pro in stomach cancer patients compared with 41.2% and 11.1% in controls (P = 0.032). We observed a significantly higher distribution of Pro/Pro genotype in the patient with stomach cancer, especially in the patients with EGC than control. Also in comparison between AGC and EGC, the frequency of Arg/Arg genotype was higher than the frequency of AGC, statistically (P = 0.038).
The reason for the tissue-specific difference of the germ line p53 polymorphism was unknown, though an association with histopathologic grading was suggested for gastric cancer[16]. In this study, we divided two groups whether signet ring cell type or not. Generally, we knew that the stomach cancer with signet ring type was highly malignant and had worse prognosis than other cell types. There was statistically significant difference that the patients with signet ring cell type had a higher ratio of Pro/Pro genotype than non-signet ring cell type (P = 0.031, Table 3). So the association with histopathologic grading may suggest that germ line p53 polymorphism is involved in survival as a clinical prognostic factor as well as cancer susceptibility. Further studies are needed to examine this possibility.
| Histopathological classification (n) | p53 genotypes | ||
| Arg/Arg | Arg/Pro | Pro/Pro | |
| Control (216) | 89/216(41.2) | 103/216(47.7) | 24/216(11.1) |
| Differentiation | |||
| Well to poorly (246) | 88/292 (30.1) | 151/292 (51.7) | 53/292 (18.2) |
| Signet ring cell (46) | 17/46 (37.0) | 11/46 (23.0) | 18/46 (39.1)ab |
| Tumor histologic type | |||
| Intestinal type (140) | 48/140 (34.2) | 60/140 (42.8) | 31/140 (22.1) |
| Diffuse type (152) | 53/152 (34.8) | 66/152 (43.4) | 34/152 (22.4) |
There were several explanations about the role of different genotypes. First, transcriptional properties are different. It has been shown that the Arg/Arg and Pro/Pro variants differ in binding activity at transcription, to activate transcription, and to induce apoptosis or cell cycle arrest[20]. The p53 Arg/Arg variant induces apoptosis with faster kinetics more efficiently than the p53 Pro/Pro variant and the p53 Arg/Arg variant is a better inducer of transcription[19]. Namely, if there was a high proportion of Arg/Arg genotype in stomach cancer, it induced the efficient apoptosis or cell cycle arrest, and then it could be better prognosis. Second, wild-type p53 protein is rapidly degraded, and has a short half-life and low intracellular levels. Stabilization of the protein following an appropriate stimulus such as DNA damage is a physical regulation to increase function[6]. Different structure of p53 proteins resulting from a substitution of Pro for Arg at codon 72 may have different functions in the responsiveness to different stimuli caused by diverse carcinogens such as H pylori, dietary, or nutritional deficiency. The reaction of Pro genotype and various carcinogens may have more carcinogenic properties. H pylori is the well-known cause of chronic gastritis, gastro-duodenal ulcer, and gastric cancer. Some reports studied the relationship between p53 polymorphism and H pylori and found the genotypic frequency of p53 was similar between cases and controls[21,22]. We cannot confirm results of H pylori status of all patients with stomach cancer in this study, we cannot represent the relationship between H pylori and p53 polymorphism.
In conclusion, the Pro/Pro genotype of the p53 codon 72 polymorphism carries a higher risk for gastric cancer in general (3.9-fold) and is also associated with a higher risk for EGC (5.25-fold) than AGC. Although this finding is provocative, it should be considered preliminary because of the limited sample size. Clearly, a well-designed follow-up study with a larger number of samples is needed to confirm these findings.
S- Editor Wang J L- Editor Alpini GD E- Editor Bi L
| 1. | Stadtlander CT, Waterbor JW. Molecular epidemiology, pathogenesis and prevention of gastric cancer. Carcinogenesis. 1999;20:2195-2208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 133] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Hotz J, Goebell H. Epidemiology and pathogenesis of gastric cancer. J. Meyer and H.J. Schmoll. Gastric Carcinoma. New York: Springer-Verlag 1989; 3-15. [DOI] [Full Text] |
| 3. | Tahara E. Molecular mechanism of stomach carcinogenesis. J Cancer Res Clin Oncol. 1993;119:265-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 231] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Lane DP, Crawford LV. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979;278:261-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1625] [Cited by in RCA: 1724] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 5. | Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3502] [Cited by in RCA: 3561] [Article Influence: 107.9] [Reference Citation Analysis (0)] |
| 6. | Brachmann RK. p53 mutants: the achilles' heel of human cancers? Cell Cycle. 2004;3:1030-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Zhang ZW, Farthing MJ. Molecular mechanisms of H. pylori associated gastric carcinogenesis. World J Gastroenterol. 1999;5:369-374. [PubMed] |
| 8. | Shepherd T, Tolbert D, Benedetti J, Macdonald J, Stemmermann G, Wiest J, DeVoe G, Miller MA, Wang J, Noffsinger A. Alterations in exon 4 of the p53 gene in gastric carcinoma. Gastroenterology. 2000;118:1039-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Monique GCT, van Oijen, Slootweg PJ. Gain-of-function mutation in the tumor suppressor gene p53. Clin Cancer Res. 2000;6:2138-2145. [PubMed] |
| 10. | Yu MW, Yang SY, Chiu YH, Chiang YC, Liaw YF, Chen CJ. A p53 genetic polymorphism as a modulator of hepatocellular carcinoma risk in relation to chronic liver disease, familial tendency, and cigarette smoking in hepatitis B carriers. Hepatology. 1999;29:697-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 102] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Milner J. Flexibility: the key to p53 function? Trends Biochem Sci. 1995;20:49-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Hoe YJ, Cho HM, Chin HM, Kim W, Jeon HM. Gastric cancer susceptibility in the p53 codon 72 polymorphism. J Korean Surg Soc. 2005;69:24-30. |
| 13. | Bae DH, Kim JS, Choi SD, Sunwoo JG, Nam KH, Kim CJ. Human papillomavirus infection and polymorphism of p53 codon 72 in the uterine cervical neoplasia. Korean J Gynecol Oncol. 2003;14:281-289. |
| 14. | Perez-Perez GI, Bosques-Padilla FJ, Crosatti ML, Tijerina-Menchaca R, Garza-Gonzalez E. Role of p53 codon 72 polymorphism in the risk of development of distal gastric cancer. Scand J Gastroenterol. 2005;40:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Marin MC, Jost CA, Brooks LA, Irwin MS, O'Nions J, Tidy JA, James N, McGregor JM, Harwood CA, Yulug IG. A common polymorphism acts as an intragenic modifier of mutant p53 behaviour. Nat Genet. 2000;25:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 381] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 16. | Bong JG, Lee MH, Song KE, Kim T, Yu W. p53 gene mutation, tumor p53 protein overexpression, and serum and-p53 antibody in patients with gastric cancer. J Korean Gastric Cancer Assoc. 2003;3:206-213. |
| 17. | Wang YC, Chen CY, Chen SK, Chang YY, Lin P. p53 codon 72 polymorphism in Taiwanese lung cancer patients: association with lung cancer susceptibility and prognosis. Clin Cancer Res. 1999;5:129-134. [PubMed] |
| 18. | Wang YC, Lee HS, Chen SK, Chang YY, Chen CY. Prognostic significance of p53 codon 72 polymorphism in lung carcinomas. Eur J Cancer. 1999;35:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Zhang ZW, Newcomb P, Hollowood A, Feakins R, Moorghen M, Storey A, Farthing MJ, Alderson D, Holly J. Age-associated increase of codon 72 Arginine p53 frequency in gastric cardia and non-cardia adenocarcinoma. Clin Cancer Res. 2003;9:2151-2156. [PubMed] |
| 20. | Thomas M, Kalita A, Labrecque S, Pim D, Banks L, Matlashewski G. Two polymorphic variants of wild-type p53 differ biochemically and biologically. Mol Cell Biol. 1999;19:1092-1100. [PubMed] |
| 21. | Wu MT, Chen MC, Wu DC. Influences of lifestyle habits and p53 codon 72 and p21 codon 31 polymorphisms on gastric cancer risk in Taiwan. Cancer Lett. 2004;205:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Hiyama T, Tanaka S, Kitadai Y, Ito M, Sumii M, Yoshihara M, Shimamoto F, Haruma K, Chayama K. p53 Codon 72 polymorphism in gastric cancer susceptibility in patients with Helicobacter pylori-associated chronic gastritis. Int J Cancer. 2002;100:304-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |