Published online Oct 28, 2006. doi: 10.3748/wjg.v12.i40.6464
Revised: July 12, 2006
Accepted: September 4, 2006
Published online: October 28, 2006
AIM: To study whether matrix metalloproteinase-9 (MMP-9) is a key factor in epithelial damage in the dextran sodium sulphate (DSS) model of colitis in mice.
METHODS: MMP-9-deficient and wild-type (wt) mice were given 5% DSS in drinking water for 5 d followed by recovery up to 7 d. On d 5 and 12 after induction of colitis, gelatinases, MMP-2 and MMP-9, were measured in homogenates of colonic tissue by zymography and Western blot, whereas tissue inhibitor of metalloproteinases (TIMPs) were measured by reverse zymography. The gelatinolytic activity was also determined in supernatants of polymorphonuclear leukocytes (PMN) isolated from mice blood. Moreover, intestinal epithelial cells were stimulated with TNF-α to study whether these cells were able to produce MMPs. Finally, colonic mucosal lesions were measured by microscopic examination.
RESULTS: On d 5 of colitis, the activity of MMP-9 was increased in homogenates of colonic tissues (0.24 ± 0.1 vs 21.3 ± 6.4, P < 0.05) and PMN from peripheral blood in wt (0.5 ± 0.1 vs 10.4 ± 0.7, P < 0.05), but not in MMP-9-deficient animals. The MMP-9 activity was also up-regulated by TNF-α in epithelial intestinal cells (2.5 ± 0.5 vs 14.7 ± 3.0, P < 0.05). Although colitis also led to increase of TIMP-1 activity, the MMP-9/TIMP-1 balance remained elevated. Finally, in the MMP-9-deficient colitic mice both the extent and severity of intestinal epithelial injury were significantly attenuated when compared with wt mice.
CONCLUSION: We conclude that DSS induced colitis is markedly attenuated in animals lacking MMP-9. This suggests that intestinal injury induced by DSS is modulated by MMP-9 and that inhibition of this gelatinase may reduce inflammation.
- Citation: Santana A, Medina C, Paz-Cabrera MC, Díaz-Gonzalez F, Farré E, Salas A, Radomski MW, Quintero E. Attenuation of dextran sodium sulphate induced colitis in matrix metalloproteinase-9 deficient mice. World J Gastroenterol 2006; 12(40): 6464-6472
- URL: https://www.wjgnet.com/1007-9327/full/v12/i40/6464.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i40.6464
Matrix metalloproteinases (MMPs) comprise a group of zinc and calcium-dependent endopeptidases that exhibit differential proteolytic activity against extracellular matrix (ECM) proteins. Based on substrate specificity, MMPs have been classically divided into collagenases, gelatinases (MMP-9 and MMP-2) and stromelysins. These proteinases are secreted as latent enzymes that require proteolytic cleavage for activation. However, a subset of MMPs, known as membrane-type MMPs (MT-MMPs) are not secreted but instead remain attached to cell surfaces and activate secreted MMPs[1,2]. The MMP activity is tightly controlled by specific endogenous inhibitors of these enzymes (TIMPs), which complex with MMPs. Four TIMPs have been described until now and TIMP-1 seems to be the most important endogenous inhibitor, which binds to activated interstitial collagenase and gelatinases[3-5].
Ulcerative colitis (UC) is a chronic inflammatory disease affecting primarily the distal colon and rectum of young adults and its etiology still remains unclear. Degradation and remodeling of the ECM is increasingly implicated in the pathogenesis of several inflammatory disorders, such as periodontal disease and rheumatoid arthritis[6-8]. In physiological conditions, MMPs act as part of the normal connective tissue turnover. However, MMPs released by diverse cells in response to several cytokines during inflammation, may lead to excess degradation of ECM and tissue injury. Previous studies have found both increased activity and expression of MMPs in colonic tissues of patients with inflammatory bowel disease (IBD)[9-13]. Moreover, MMP-9 may be a key factor responsible for accelerated breakdown of ECM in UC, because it was demonstrated that MMP-9 is abundantly expressed in patients with UC compared with controls[9]. Hence, MMP inhibition has emerged as a potential therapeutic approach in colonic inflammatory disorders[14-16]. Furthermore, we have previously demonstrated a significant up-regulation of MMP-9 expression and activity in a rat model of colitis induced by dextran sulphate sodium (DSS). In this experimental model, a synthetic MMP inhibitor (CGS-27023-A) significantly reduced the extent and severity of tissue injury[17]. However, this compound is a broad spectrum MMP inhibitor and the relative contribution of MMP-9 in this experimental model still remains unclear.
In the present investigation, we sought to determine whether MMP-9 extinction could alter intestinal damage in a mouse model of colitis induced by DSS. This model exhibits clinical and morphological features resembling human UC, including diarrhea and rectal bleeding, diffuse lesions circumscribed to the mucosa, and predominance of distal involvement of the large intestine[18]. Our findings support the conclusion that MMP-9 modulates colonic mucosal injury in this entity.
Our studies used the homozygous MMP-9-deficient male mice with FVB background and wild-type (wt) purchased from Jackson Laboratories (Ca, USA). The animals were 8 wk to 10 wk old and weighted from 25 g to 30 g. They were maintained in a restricted-access room with controlled temperature (23°C) and light-dark cycle (12 h:12 h) and were housed in rack-mounted cages with a maximum of 10 mice per cage. Mice were allowed to drink and feed ad libitum. The study was approved by the local Animal Welfare Committee and conformed to the principles of laboratory animal care and use (NIH publication 86-23).
Liver samples from selected mice were collected and genotype was verified by polymerase chain reaction (PCR) using a sense oligonucleotide primer (5’-GCATACTTGTACCGCTATGG-3’) and an antisense oligonucleotide primer (5’-TAACCGGAGGTCCAAACTGG-3’). For the neomycin cassette, we also used a sense oligonucleotide primer (5´-GAAGGGACTGGCTGCTATTG-3´) and an antisense primer (5´-AATATCACGGGTAGCCAACG-3´). All procedures were performed blind with respect to genotype.
Distal colitis was induced by oral DSS (molecular wt 40 000; ICN Biomedicals, Aurora, OH) at 5% in tap water ad libitum for 5 d in wt and MMP-9 deficient mice and then switched to water and monitored for 7 additional days. At least 15 age and sex matched wt and MMP-9 deficient mice were studied at the end of 5 d with DSS treatment and at 7 d after DSS (recovery phase), whereas 15 control wt and 15 control MMP-9 deficient mice were allowed free access to drinking water. Body weight was routinely obtained every second day and mortality was recorded at any time. Mice were euthanized by cervical dislocation on d 5 and 12 after induction of colitis. With the use of sterile equipment, a mid laparotomy was performed, the colon was removed, opened longitudinally, rinsed with sterile saline, and divided into two parts by a longitudinal section. One specimen was homogenized and stored at -20°C for MMPs, TIMPs, TNF-α and myeloperoxidase (MPO) assay. The second specimen was used for microscopic assessment of mucosal lesions.
Mice were anesthetized by an intraperitoneal injection of ketamine (100 mg/kg, Park Davis, Morris Plains, NJ). Blood samples were collected from the abdominal aorta. Neutrophils were isolated using Histopaque (Sigma Ltd, England) and erythrocyte removed using hypotonic lysis with ammonium chloride. The neutrophil count was determined by using a Neubaver hemocytometer and cell viability was assessed by trypan blue exclusion. Typically, the neutrophil preparations were > 98% pure and > 94% viable. The isolated neutrophils were washed and resuspended in HBSS with calcium and magnesium at a concentration of 5 × 109 cells/L and incubated for 12 h at 37°C. The release of MMPs was determined in the supernatant by zymography[19].
Caco-2 cells, a human intestinal epithelial cell line, were obtained from the American Type Culture Collection (ATCC, Rockville, MD). Cells were cultured in 75-cm2 flasks in a humidified atmosphere containing 5% CO2 at 37°C. Cells were grown in minimum essential medium supplemented with pyruvate, sodium bicarbonate, 20% fetal bovine serum, gentamycin sulfate (0.05 mg/mL), penicillin G (0.06 mg/mL) and streptomycin sulfate (0.01 mg/mL). The medium was changed every two days and cells were subcultured three times each week. When confluent, cells were detached using a Trypsin-EDTA solution. For the in vitro experiments, Caco-2 cells were seeded into 24-well plates (30 000 cells per well) in free serum-medium and cultured in the presence or absence of human recombinant TNF-α (1 or 10 μg/L) for 24 h. Afterwards, conditioned media was collected and stored at -20°C for the zymography assay and Western blot analysis.
Zymography: The activity of pro-MMP-9 was measured as previously described[20,21] in homogenates of colonic tissue, supernatants of purified neutrophils and Caco-2 conditioned media. Briefly, samples were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with copolymerized gelatin (0.2%; Sigma Chemical Co, St. Louis, Mo). After electrophoresis, the gels were washed with 2% Triton X-100 (2 times, 20 min each), and then incubated in development buffer (50 mmol/L Tris HCl, 200 mmol/L NaCl, 10 mmol/L CaCl2 and 1 μmol/L ZnCl2 , pH = 7.5) at 37°C overnight. Human recombinant MMP-9 and MMP-2 (Oncogene Research, Nottingham, UK), conditioned medium of HT-1080 human fibrosarcoma cells and molecular weight markers were used as standards. After incubation, gels were fixed and stained in 40% methanol, 10% acetic acid and 0.1% (wt/v) Coomassie Blue for 1 h and then de-stained. The low availability of active MMP-9 seen in the zymographies may be due to its high level of instability and the removal of active enzyme during the washing of specimens, as it has been previously suggested[22].
Reverse zymography: The TIMP-1 activity was ana-lyzed in homogenates of colonic tissues, as previously described[23]. Briefly, samples were subjected to 15% SDS-PAGE with copolymerized 0.2% gelatin and human recombinant MMP-2 (160 μg/L). Human recombinant TIMP-1 was used as the internal standard. TIMP-1 activity was identified by inhibition of gelatinolysis when compared with the standard.
Western-blot: Western-blot analysis of MMP-9 was performed in homogenates of colonic tissue and Caco-2 conditioned media, as previously described[20]. Samples were denatured and loaded (50 μg/lane) onto 10% (vol/vol) polyacrylamide gels. Following electrophoresis, proteins were transferred onto a nitrocellulose membrane (Protran, Schleider&Schuell) and detected using a rabbit monoclonal anti-MMP-9 antibody (Chemicon, Ca, USA) at 1:1000 concentration and a chemiluminescent substrate (Pierce, Rockford, USA). To account for the inter- blot variations in MMP immunoreactivity, an internal standard (conditioned medium of HT-1080 cells, and recombinant MMP-9) was used[18]. Western Blotting with monoclonal anti-beta-actin (Sigma Chemical Co, St. Louis, Mo) was performed as an internal control.
Bands quantification: Clear bands were analyzed using a calibrated densitometer (GS-800, BioRad) and Quantity One Quantitation analysis software (BioRad, version 4). Each band was measured in terms of Optical Density Units of trace quantity (ODu) × mm.
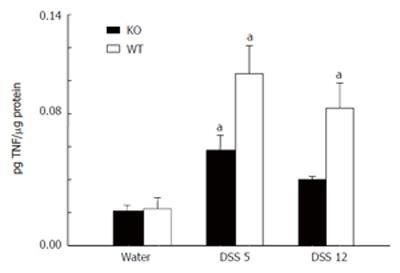
Quantification of TNF-αby ELISA: TNF-α expression was assayed in colonic tissues. Fragments of colon (40-50 mg) from the proximal region of the rectum were collected from wt and KO animals treated as described above. Afterwards, colon tissues were placed in 500 mL ice-cold PBS containing 20 μL of a proteinase inhibitor cocktail (Roche Diagnostic GmbH, San Francisco, CA) and immediately sonicated. After 10 min in ice, cellular debris was removed by centrifugation at 10 000 g for 10 min at 4°C and the protein content of the supernatant was assayed by Bradford's method. The specific ELISAs for mouse TNF-α used in these experiments were purchased from BD Biosciences (San Diego, CA). Murine TNF-α concentrations in tissue extracts were assayed in duplicate wells following the manufacturer’s recommendations. The sensitivity of ELISA for TNF-α in our laboratory was 20 ng/L. Data were expressed as pg TNF-α/μg of total protein.
MPO assay: For the MPO activity assay[24], the colonic specimen was homogenized in 2 mL phosphate-buffered saline, using a Tissue Tearor (model 985-370, Biospec, Racine, WI), and centrifuged. The pellets were again homogenized in an equivalent volume of phosphate buffer (50 mmol/L, pH 6) containing 0.5% hexadecyltrimethylammonium bromide (Sigma) and 5 mmol/L EDTA, sonicated three times for 30 s each time (Labasonic 2000, Braun), and centrifuged. Supernatants were used for determination of tissue MPO activity by a kinetic method. One unit of enzyme activity is defined as the amount of MPO that degrades 1 mmol of peroxide per minute at 25°C.
Colonic specimens were fixed in formalin and coded for blind microscopic measurement of mucosal lesions. Samples were embedded in paraffin using a “Swiss roll” technique, and longitudinal sections from cecum to rectum were prepared and stained with hematoxylin and eosin. This procedure allows the examination of the entire specimen from cecum to rectum in every section. Two pathologists who were unaware of the treatment measured the extent of mucosal surface involved by crypt lesions as a percentage of the total colonic surface. For each specimen, a crypt damage score was obtained by summation of the products of each grade of crypt damage times the extent of mucosal surface involved by this grade of damage (Table 1). In addition, the specimens were graded 0 to 3 for inflammation (acute + chronic), regenerative changes (hyperplastic epithelium) and crypt distortion (distorted epithelium) using the criteria described by Cooper et al[25]. Acute inflammation scores evaluated of the presence of polymorphonuclear neutrophils in the infiltrate, whereas chronic inflammation scores evaluate the presence of mononuclear cells.
| A: Grade of crypt lesion | Score |
| Intact crypt | 0 |
| Grade 1: Loss of the basal third | 1 |
| Grade 2: Loss of two thirds | 2 |
| Grade 3: Loss of entire crypt | 3 |
| Grade 4: Erosion | 4 |
| B: Extension of each grade of crypt lesion as % of total mucosal surface | 0-100 |
| Crypt lesion score | Σ (A × B) |
| Grade of inflammation, epithelial regeneration or crypt distortion | Score |
| Absent | 0 |
| Mild | 1 |
| Moderate | 2 |
| Severe | 3 |
Results are presented as mean ± SE. The statistical difference between means was determined using one way analysis of variance for overall comparison and the Student-Newman-Keuls test as post-test for single comparisons. The mortality data were analyzed by Kaplan-Meier survival curves.
Exposure to 5% DSS in drinking water for 5 d induced diarrhea and rectal bleeding in all mice. Furthermore, DSS treatment resulted in a significant increase in MPO activity in the colonic tissues of both MMP-9 deficient mice (colitic: 2.5 ± 0.82 vs control: 0.3 ± 0.05 mU/mg protein, P < 0.05) and wt (colitic: 1.95 ± 0.67 vs control: 0.25 ± 0.06 mU/mg protein, P < 0.05). Histological examination of colonic sections revealed that administration of DSS resulted in epithelium injury and loss of normal crypt architecture, with some areas of erosion. Mixed infiltrate of neutrophils and mononuclear cells was observed in the lamina propria and submucosa. The muscularis propria was not involved. These changes were diffuse but predominated at distal parts of the colon. Histological examination revealed no changes in colons from normal control mice.
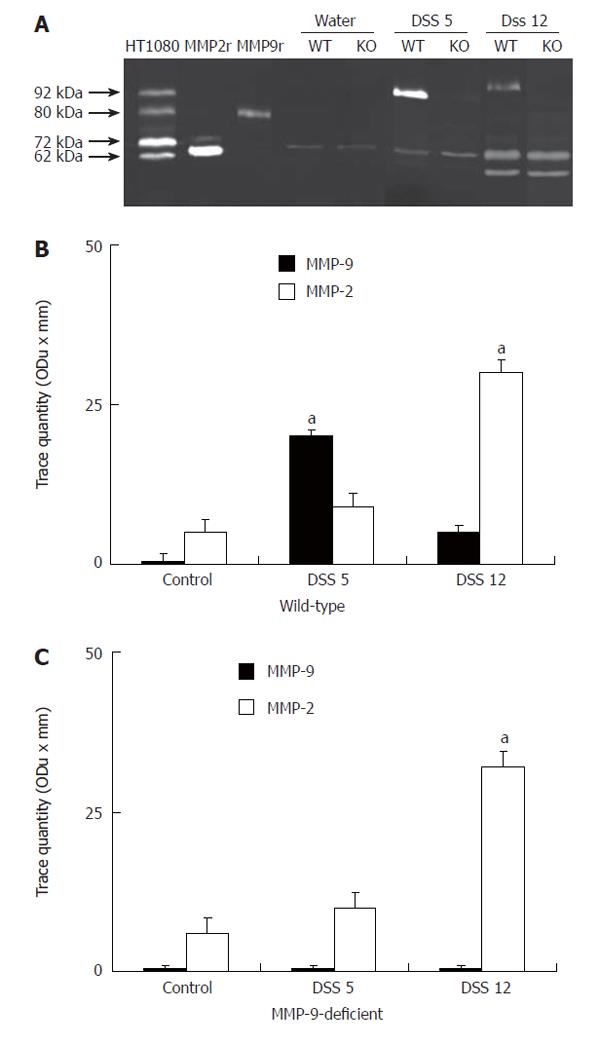
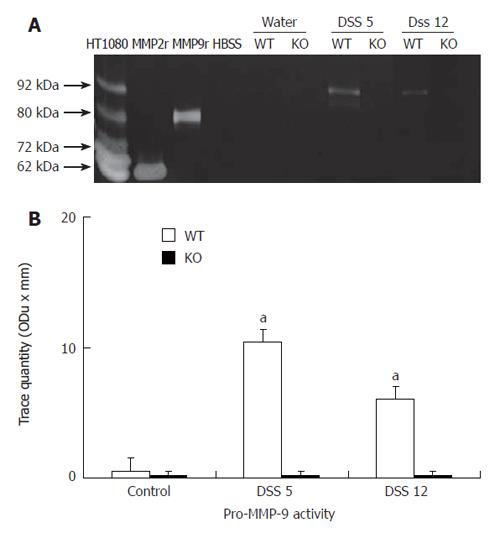
Zymographies revealed that MMP-2 but not pro-MMP-9, was a dominant gelatinase in colonic homogenates from non-colitic animals in both wt and deficient animals (Figure 1A). However, DSS treatment resulted in a significant up-regulation of pro-MMP-9, but not MMP-2 activity in colitic wt animals in comparison to deficient animals on d 5 after the induction of colitis (Figure 1A and B). On d 12, pro-MMP-9 activity was still up-regulated in wt animals, although to a less extent than in d 5. In addition, there was an up-regulation of MMP-2 in both groups (Figure 1A and B). In peripheral neutrophils isolated from wt animals pro-MMP-9 activity was also markedly enhanced on d 5 and, to a lesser extent, on d 12 following the induction of colitis, but not from MMP-9 deficient mice (Figure 2A and B). By contrast, no MMP-2 activity was shown in neutrophils isolated from any animal group in the presence or absence of DSS treatment.
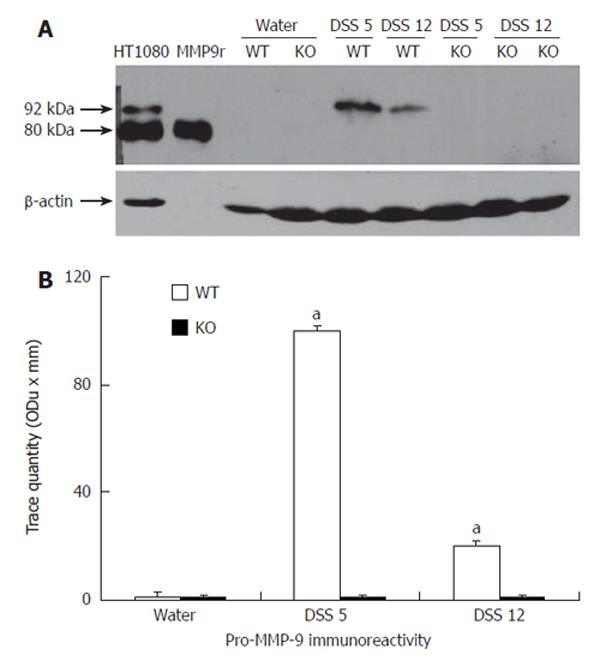
As shown by the immunoreactive band migrating at 92 kDa, a significant up-regulation of pro-MMP-9 was observed in wt, but not in controls or MMP-9 deficient mice, on d 5 and 12 following the induction of colitis (Figure 3).
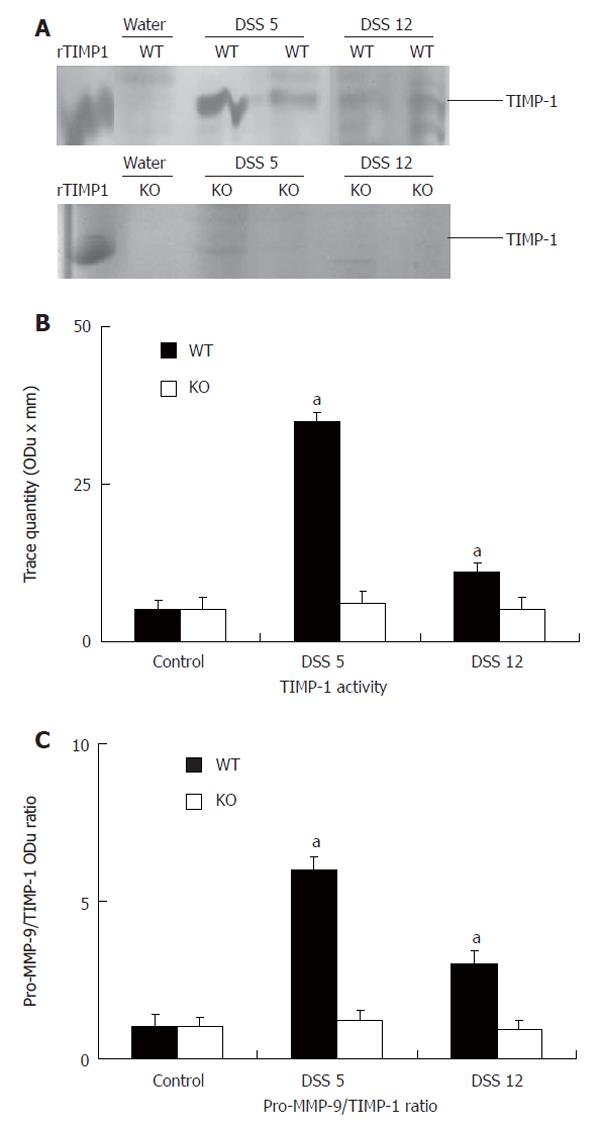
Colitis resulted in a significant up-regulation of TIMP-1 in wt animals receiving DSS compared to controls on d 5 and this increase remained elevated on d 12 after induction of colitis (Figure 4A and B). In wt mice the pro-MMP-9/TIMP-1 ratio was significantly higher (P < 0.05) than in control mice on d 5 and 12 after the induction of colitis. In addition, the pro-MMP-9/TIMP-1 ratio was not altered by colitis in MMP-9-deficient mice (Figure 4C).
TNF-αContent in DSS Colitis
Figure 5 shows that wt and MMP-9-deficient animals exposed to DSS for during 5 d showed a significant increase in TNF-α concentration as compared with non-colitic animals. In addition, the TNF-α level remained elevated in wt animals on d 12 after colitis induction, whereas returned to base line levels in MMP-9 deficient animals.
Effect of TNF-αTreatment
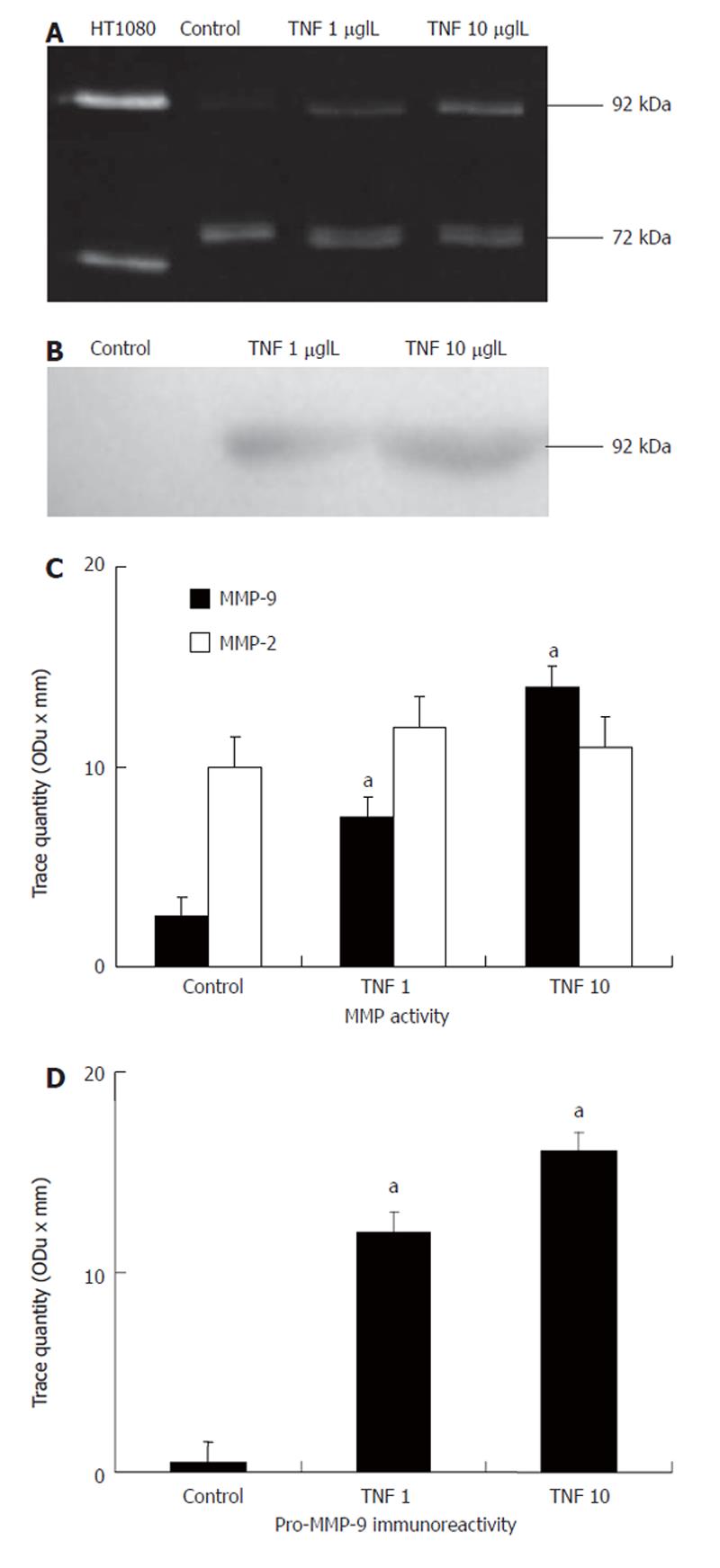
Since in DSS-induced colitis the predominant lesions are circumscribed to the mucosa, we measured the MMP activity in intestinal epithelial (Caco-2) cells in response to TNF-α. Zymographies showed that, under basal conditions, MMP-2 was the predominant gelatinase in Caco-2 cells. Interestingly, TNF-α treatment resulted in a dose-dependent significant increase of pro-MMP-9 activity whereas MMP-2 activity remained unchanged (Figure 6A and C). In addition, Western blot analysis corroborated that TNF-α treatment significantly increased pro-MMP-9 immunoreactivity in intestinal epithelial cells (Figure 6B and D).
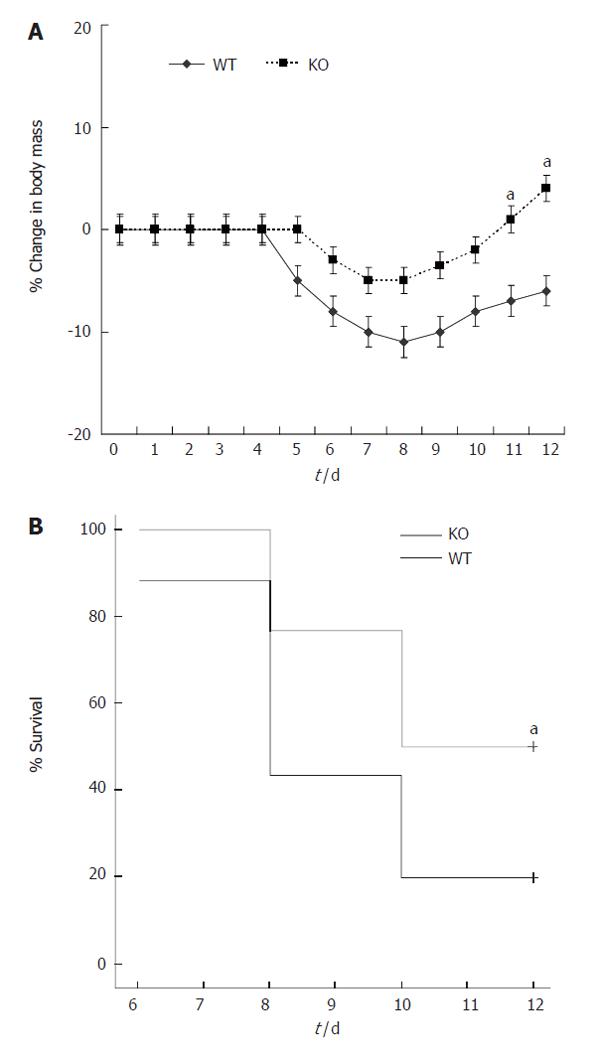
Both wt and MMP-9-deficient mice developed diarrhea and rectal bleeding with DSS. Percent loss of body weight is shown in Figure 7A. Neither deficient nor wt mice showed major loss of body weight during DSS treatment. However, during the recovery phase, wt mice showed significant weight loss. In addition, the mortality was significantly higher in wt than in MMP-9 KO mice (Figure 7B). The mean of severity of crypt damage, inflammation, epithelial regeneration and crypt distortion scores are summarized in Table 2. MMP-9 deficient mice showed a significant decrease in the crypt damage scores when compared with wt mice on d 5 and 12 after colitis induction. Moreover, deficient mice showed significantly lower scores of acute and chronic inflammation on d 12. Epithelial regeneration and crypt distortion scores were almost absent on d 5, and no differences were found between both groups at any time.
The etiology and the cascade of the events resulting in intestinal injury of UC patients still remain unclear. There is growing evidence that MMPs are implicated in tissue remodeling and destruction associated with several inflammatory conditions[6-8], including IBD[9-13]. Moreover, increased gelatinase activity has been shown in diverse animal experimental models of colitis[17,26] Our study revealed that MMP-9-deficient animals showed improved survival and indices of epithelial injury, suggesting that MMP-9 is a key factor in tissue damage in DSS-induced colitis.
The destructive effect of MMPs in the gut has been shown by several in vitro studies[27-29]. In addition, previous reports have found increased levels of MMP-9 in colonic tissues of patients with IBD. For instance, Bailey et al[30] have shown an increased MMP-9 protein in patients with IBD by immunohistochemical techniques. Furthermore, Baugh et al have found that MMP-9 activity measured by zymography was the most abundant MMP expressed in intestinal tissues of patients with UC compared with normal controls[9]. Moreover, Tartlon et al[26] have also shown increased gelatinase activity in a transmural experimental model of colitis induced in immunodeficient mice. Finally, we have previously found that MMP-9 is up-regulated in intestinal tissues from rats with DSS-induced colitis[17]. Our present study shows that DSS treatment induced an increased activity and expression of pro-MMP-9 in wt mice compared to the MMP-9 deficient group. It could be surprising that DSS did not induce the active form of MMP-9, however, the low availability of this enzyme may be due to the removal of the active form of MMP-9 during the washing of specimens, as it has been previously suggested[22]. Our data also show that colitis induced by DSS was enhanced in wt animals. In fact, histological evidence of less mucosal damage was observed in MMP-9 deficient animals on d 5 and 12 after induction of colitis. In addition, the higher weight loss and mortality of wt mice compared with deficient animals supports this hypothesis. In our experiments the indices of DSS-induced colitic injury correlated well with pro-MMP-9, but not with MMP-2 up-regulation. By contrast, whereas MMP-2 activity was elevated during the recovery phase of colitis, the activity and expression of pro-MMP-9 decreased on d 12 after colitis induction. These data suggest that MMP-9 is mainly implicated in mucosal injury at the early stage of colitis, whereas MMP-2 seems to be linked to mucosal repair, although MMP-2 deficient animals should be tested to confirm this hypothesis. Therefore, further investigation is warranted.
In many inflammatory conditions, the excess degra-dation of ECM may result from an imbalance between MMP and TIMP activity[31]. Since TIMP-1 seems to be the major inhibitor of gelatinase activity[3] we studied the activity of this inhibitor in DSS-induced colitis. We found that TIMP-1 was increased in colitic wt animals on d 5 after induction of colitis in comparison with non-colitic mice. However, our results showed an imbalance between pro-MMP-9 and TIMP-1 activity in colitic wt animals in favour of MMP-9, suggesting that the increase of TIMP-1 was insufficient in preventing pro-MMP-9 from exerting its biologic effects in DSS-induced colitis. Interestingly, in MMP-9 deficient animals DSS treatment did not increase TIMP-1 activity, which could suggest an adapted regulatory response of this endogenous inhibitor to the levels of pro-MMP-9. Our results are consistent with previous studies in human IBD where an imbalance between MMPs and their endogenous inhibitors has been shown[10,12].
We next studied the cellular origin of MMP-9 in DSS-induced colitis. MMP-9 released from neutrophils could be an important factor for transmigration and proteolysis of ECM in several inflammatory conditions contributing to tissue injury[19,32]. We found that pro-MMP-9 is expressed by neutrophils only in colitic wt animals. However, DSS treatment did not induce any significant difference in MPO activity and inflammation score (markers of neutrophilic infiltration) between wt and MMP-9 deficient animals on d 5. This is consistent with the fact that inflammation is thought to be a secondary event that follows epithelial damage in this experimental model of colitis[25]. Therefore, it seems reasonable that other cells may also generate MMP-9 contributing to crypt damage during the initial phase of DSS-induced colitis. Intestinal epithelium plays an important role in the immunomodulatory response of the intestinal mucosa[33,34]. Indeed, the loss of intestinal epithelium integrity could lead to an interaction between the luminal antigenic stimuli with the mucosal immune system, resulting in intestinal inflammation[35-37]. We used a human intestinal epithelial cell line to confirm whether MMP-9 and MMP-2 are up-regulated by inflammatory stimuli, as previously described[38]. Consistent with the colonic tissue samples, we found that TNF-α treatment only resulted in up-regulation of pro-MMP-9 activity in intestinal epithelial cells. These data therefore suggest that MMP-9 could be released from inflamed intestinal epithelial cells with the subsequent loss of mucosal integrity, thus facilitating the penetration of inflammatory cells such as PMN into inflamed tissue in the late inflammatory phase of DSS-induced colitis. Our results are consistent with a recent study where epithelial MMP-9 has been shown as an important factor for tissue damage using the Caco-2 intestinal cell line, since MMP-9 inhibited cell attachment and wound healing in an in vitro model[39]. In addition, epithelial protection in MMP-9-deficient mice during the early phase of colitis could also account for a mitigated inflammatory response in the late phase of this experimental model. In fact, only on d 12 after DSS administration MMP-9- deficient animals showed lower inflammation scores than wt animals. These results are consistent with our previous study, where MMP inhibition with CGS-27023-A treatment in rats with DSS-induced colitis significantly reduced the inflammation scores only during the recovery phase of the colitis[17].
In conclusion, this study presents direct evidence that DSS-induced colitis is attenuated in mice deficient in MMP-9. The reduction in inflammatory indices and pathological scoring suggests a link between MMP-9 and DSS-induced colitis that might help elucidate the physiopathology of UC. These results suggest that selective inhibition of this gelatinase could be a novel therapeutic strategy for patients with UC.
S- Editor Pan BR L- Editor Alpini GD E- Editor Ma WH
| 1. | Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135-1149. [PubMed] |
| 2. | Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2922] [Cited by in RCA: 2886] [Article Influence: 120.3] [Reference Citation Analysis (0)] |
| 3. | Carmichael DF, Sommer A, Thompson RC, Anderson DC, Smith CG, Welgus HG, Stricklin GP. Primary structure and cDNA cloning of human fibroblast collagenase inhibitor. Proc Natl Acad Sci USA. 1986;83:2407-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 206] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Greene J, Wang M, Liu YE, Raymond LA, Rosen C, Shi YE. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem. 1996;271:30375-30380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 387] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Leco KJ, Khokha R, Pavloff N, Hawkes SP, Edwards DR. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is an extracellular matrix-associated protein with a distinctive pattern of expression in mouse cells and tissues. J Biol Chem. 1994;269:9352-9360. [PubMed] |
| 6. | Ruwanpura SM, Noguchi K, Ishikawa I. Prostaglandin E2 regulates interleukin-1beta-induced matrix metallopro-teinase-3 production in human gingival fibroblasts. J Dent Res. 2004;83:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Kim H, Kim WJ, Jeon ST, Koh EM, Cha HS, Ahn KS, Lee WH. Cyclophilin A may contribute to the inflammatory processes in rheumatoid arthritis through induction of matrix degrading enzymes and inflammatory cytokines from macrophages. Clin Immunol. 2005;116:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Ishikawa T, Nishigaki F, Miyata S, Hirayama Y, Minoura K, Imanishi J, Neya M, Mizutani T, Imamura Y, Ohkubo Y. Prevention of progressive joint destruction in adjuvant induced arthritis in rats by a novel matrix metalloproteinase inhibitor, FR217840. Eur J Pharmacol. 2005;508:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Baugh MD, Perry MJ, Hollander AP, Davies DR, Cross SS, Lobo AJ, Taylor CJ, Evans GS. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology. 1999;117:814-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 255] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Heuschkel RB, MacDonald TT, Monteleone G, Bajaj-Elliott M, Smith JA, Pender SL. Imbalance of stromelysin-1 and TIMP-1 in the mucosal lesions of children with inflammatory bowel disease. Gut. 2000;47:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Stallmach A, Chan CC, Ecker KW, Feifel G, Herbst H, Schuppan D, Zeitz M. Comparable expression of matrix metalloproteinases 1 and 2 in pouchitis and ulcerative colitis. Gut. 2000;47:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut. 2000;47:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 290] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 13. | Kirkegaard T, Hansen A, Bruun E, Brynskov J. Expression and localisation of matrix metalloproteinases and their natural inhibitors in fistulae of patients with Crohn's disease. Gut. 2004;53:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 164] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Medina C, Videla S, Radomski A, Radomski M, Antolin M, Guarner F, Vilaseca J, Salas A, Malagelada JR. Therapeutic effect of phenantroline in two rat models of inflammatory bowel disease. Scand J Gastroenterol. 2001;36:1314-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Medina C, Santana A, Llopis M, Paz-Cabrera MC, Antolin M, Mourelle M, Guarner F, Vilaseca J, Gonzalez C, Salas A. Induction of colonic transmural inflammation by Bacteroides fragilis: implication of matrix metalloproteinases. Inflamm Bowel Dis. 2005;11:99-105. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Sykes AP, Bhogal R, Brampton C, Chander C, Whelan C, Parsons ME, Bird J. The effect of an inhibitor of matrix metalloproteinases on colonic inflammation in a trinitrobenzenesulphonic acid rat model of inflammatory bowel disease. Aliment Pharmacol Ther. 1999;13:1535-1542. [RCA] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Medina C, Videla S, Radomski A, Radomski MW, Antolín M, Guarner F, Vilaseca J, Salas A, Malagelada JR. Increased activity and expression of matrix metalloproteinase-9 in a rat model of distal colitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G116-G122. [PubMed] |
| 18. | Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694-702. [PubMed] |
| 19. | Keck T, Balcom JH 4th, Fernandez-del Castillo C, Antoniu BA, Warshaw AL. Matrix metalloproteinase-9 promotes neutrophil migration and alveolar capillary leakage in pancreatitis-associated lung injury in the rat. Gastroenterology. 2002;122:188-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Fernandez-Patron C, Martinez-Cuesta MA, Salas E, Sawicki G, Wozniak M, Radomski MW, Davidge ST. Differential regulation of platelet aggregation by matrix metalloproteinases-9 and -2. Thromb Haemost. 1999;82:1730-1735. [PubMed] |
| 21. | Radomski A, Sawicki G, Olson DM, Radomski MW. The role of nitric oxide and metalloproteinases in the pathogenesis of hyperoxia-induced lung injury in newborn rats. Br J Pharmacol. 1998;125:1455-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Deleve LD, Wang X, Tsai J, Kanel G, Strasberg S, Tokes ZA. Sinusoidal obstruction syndrome (veno-occlusive disease) in the rat is prevented by matrix metalloproteinase inhibition. Gastroenterology. 2003;125:882-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Oliver GW, Leferson JD, Stetler-Stevenson WG, Kleiner DE. Quantitative reverse zymography: analysis of picogram amounts of metalloproteinase inhibitors using gelatinase A and B reverse zymograms. Anal Biochem. 1997;244:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 128] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Schierwagen C, Bylund-Fellenius AC, Lundberg C. Improved method for quantification of tissue PMN accumulation measured by myeloperoxidase activity. J Pharmacol Methods. 1990;23:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 268] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 25. | Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238-249. [PubMed] |
| 26. | Tarlton JF, Whiting CV, Tunmore D, Bregenholt S, Reimann J, Claesson MH, Bland PW. The role of up-regulated serine proteases and matrix metalloproteinases in the pathogenesis of a murine model of colitis. Am J Pathol. 2000;157:1927-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Pender SL, Tickle SP, Docherty AJ, Howie D, Wathen NC, MacDonald TT. A major role for matrix metalloproteinases in T cell injury in the gut. J Immunol. 1997;158:1582-1590. [PubMed] |
| 28. | Pender SL, Fell JM, Chamow SM, Ashkenazi A, MacDonald TT. A p55 TNF receptor immunoadhesin prevents T cell-mediated intestinal injury by inhibiting matrix metalloproteinase production. J Immunol. 1998;160:4098-4103. [PubMed] |
| 29. | Pender SL, Breese EJ, Gunther U, Howie D, Wathen NC, Schuppan D, MacDonald TT. Suppression of T cell-mediated injury in human gut by interleukin 10: role of matrix metalloproteinases. Gastroenterology. 1998;115:573-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Bailey CJ, Hembry RM, Alexander A, Irving MH, Grant ME, Shuttleworth CA. Distribution of the matrix metalloproteinases stromelysin, gelatinases A and B, and collagenase in Crohn's disease and normal intestine. J Clin Pathol. 1994;47:113-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 110] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 859] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 32. | Delclaux C, Delacourt C, D'Ortho MP, Boyer V, Lafuma C, Harf A. Role of gelatinase B and elastase in human polymorphonuclear neutrophil migration across basement membrane. Am J Respir Cell Mol Biol. 1996;14:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 307] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 33. | Reinecker HC, Podolsky DK. Human intestinal epithelial cells express functional cytokine receptors sharing the common gamma c chain of the interleukin 2 receptor. Proc Natl Acad Sci USA. 1995;92:8353-8357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff MF. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 880] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 35. | García-Lafuente A, Antolín M, Guarner F, Crespo E, Salas A, Forcada P, Laguarda M, Gavaldá J, Baena JA, Vilaseca J. Incrimination of anaerobic bacteria in the induction of experimental colitis. Am J Physiol. 1997;272:G10-G15. [PubMed] |
| 36. | Qian BF, Tonkonogy SL, Hoentjen F, Dieleman LA, Sartor RB. Dysregulated luminal bacterial antigen-specific T-cell responses and antigen-presenting cell function in HLA-B27 transgenic rats with chronic colitis. Immunology. 2005;116:112-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Rath HC, Schultz M, Freitag R, Dieleman LA, Li F, Linde HJ, Schölmerich J, Sartor RB. Different subsets of enteric bacteria induce and perpetuate experimental colitis in rats and mice. Infect Immun. 2001;69:2277-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 241] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 38. | Su CG, Wen X, Bailey ST, Jiang W, Rangwala SM, Keilbaugh SA, Flanigan A, Murthy S, Lazar MA, Wu GD. A novel therapy for colitis utilizing PPAR-gamma ligands to inhibit the epithelial inflammatory response. J Clin Invest. 1999;104:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 611] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 39. | Castaneda FE, Walia B, Vijay-Kumar M, Patel NR, Roser S, Kolachala VL, Rojas M, Wang L, Oprea G, Garg P. Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: central role of epithelial-derived MMP. Gastroenterology. 2005;129:1991-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 219] [Article Influence: 11.0] [Reference Citation Analysis (0)] |