Published online Oct 28, 2006. doi: 10.3748/wjg.v12.i40.6458
Revised: February 18, 2006
Accepted: April 21, 2006
Published online: October 28, 2006
AIM: To determine the effects of prophylactic peroxi-some proliferator-activated receptor (PPARγ) agonist administration in an experimental model of post-endoscopic retrograde cholangiopancreatography (post-ERCP) acute pancreatitis.
METHODS: Post-ERCP pancreatitis was induced in male Wistar rats by infusion of contrast medium into the pancreatic duct. In additional group, rosiglitazone, a PPARγ agonist, was administered 1 h before infusion of contrast medium. Plasma and pancreas samples were obtained 6 h after the infusion.
RESULTS: Infusion of contrast medium into the pan-creatic duct resulted in an inflammatory process characterized by increased lipase levels in plasma, and edema and myeloperoxidase activity (MPO) in pancreas. This result correlated with the activation of nuclear factor κB (NFκB) and the inducible NO synthase (iNOS) expression in pancreatic cells. Rosiglitazone reduced the increase in lipase and the level of edema and the increase in myeloperoxidase as well as the activation of NFκB and iNOS expression.
CONCLUSION: A single oral dose of rosiglitazone, given 1 h before post-ERCP pancreatitis induction is effective in reducing the severity of the subsequent inflammatory process. The protective effect of rosiglitazone was associated with NFκB inhibition and the blockage of leukocyte infiltration in pancreas.
- Citation: Folch-Puy E, Granell S, Iovanna JL, Barthet M, Closa D. Peroxisome proliferator-activated receptor γ agonist reduces the severity of post-ERCP pancreatitis in rats. World J Gastroenterol 2006; 12(40): 6458-6463
- URL: https://www.wjgnet.com/1007-9327/full/v12/i40/6458.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i40.6458
Acute pancreatitis is one of the major and serious complications after diagnostic or therapeutic endoscopic retrograde cholangiopancreatography (ERCP). Despite the technical improvements of recent years and the experience of endoscopists, the incidence has not decreased and it ranges from 1% to 10% of patients[1]. The most severe forms of pancreatitis, with pancreatic necrosis, multi-organ failure and even death, occurs in 0.3%-0.6% of patients, but a silent increase in serum pancreatic enzymes could be observed in up to 70% of patients[2]. The triggering mechanism of the inflammatory response remains unclear and different pharmacological agents has been tested to prevent post-ERCP pancreatitis including anti-inflammatory steroids, somatostatin analogs, heparin, protease inhibitors and anti-inflammatory cytokines[3-6]. Only few drugs showed some efficacy to prevent post-ERCP pancreatitis like recently nitrate therapy by decreasing the pancreatic ductal pressure or diclofenac by lowering inflammatory process[7,8]. Therefore, the availability of effective drugs and strategy of chemoprevention remain as unsettled points in the pharmacological prophylaxis of post-ERCP pancreatitis[5-8], leading to the apparition of endoscopic procedure such as pancreatic sphincterotomy to try to decrease the risk of post-ERCP pancreatitis[9].
Recent evidences indicate an important role for the peroxisome proliferator-activated receptors (PPARs) in the regulation of both inflammation and lipid metabolism[10]. In particular, it has been reported, using an experimental model of cerulein-induced pancreatitis, that the pancreatic inflammation and tissue injury was markedly reduced by the administration of PPARγ agonists[11]. PPARγ is a member of the nuclear hormone receptor superfamily originally reported to be expressed at high levels in adipose tissue and to play a critical role in adipocyte differentiation, glucose metabolism and lipid storage. In an experimental model of intestinal ischemia-reperfusion, a more severe injury was observed in PPARγ-deficient mice and protection against local and remote tissue injury in mice treated with a PPARγ-activating ligand[12]. Then, it has been demonstrated that PPARγ ligands can inhibit the inflammatory response by decreasing IL-6, IL-1β, TNFα and the inducible NO synthase (iNOS) by interfering with nuclear factor κB (NFκB) and AP1[13,14]. The aim of this study was to investigate the efficacy of prophylactic PPARγ agonist treatment in reducing the pancreatic damage in an experimental post-ERCP acute pancreatitis model.
Reagents for SDS-PAGE and nitrocellulose membranes were from Amersham Pharmacia (Buckinghamshire, England). Antibodies against p65, PPARγ and Histone H1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), antibody against iNOS was obtained from BD Transduction (Heildelberg, Germany), antibody against β-actin and the secondary antibody linked to horseradish peroxidase were from Sigma Chemicals (St Louis, MO). The following reagents were obtained from Sigma Chemicals (St Louis, MO): NaVO3, NaF, Nonidet P40, ethidium bromide, Hexadecyltrimethylammonium bromide, Tetramethylbenzidine, DMSO, H2O2.
Male Wistar rats (250-300 g) were used for all experiments. Animals were housed in light-dark cycle regulated, air conditioned (23°C) and air humity (60%) animal quarters, given free access to drinking water and standard food pellets until 12 h prior to the experiment, at which point food was withdrawn. Animal care was in compliance with the European Community (Directive 86/609/EEC) for the use of experimental animals and the institutional committee of animal care and research approved it. Rats were anaesthetized with ip injection of sodium pentobarbital (10 μL/kg). The biliopancreatic duct was cannulated through the duodenum and the hepatic duct was closed by a small bulldog clamp. Post-ERCP pancreatitis was induced by retrograde infusion into the biliopancreatic duct of low osmolarity contrast medium Meglumine/Sodium Ioxaglate (Hexabrix 320) in a volume of 10 μL/kg using a Harvard '22' infusion pump (Harvard Instruments, Edenbridge, UK). Control animals were subjected to anesthesia and laparotomy[15].
In the first set of experiments, we evaluated the severity of pancreatic damage and tissue inflammation after the infusion of contrast medium into the pancreatic duct. For this purpose, rats (n = 6 for each group) were sacrificed at 0, 3, 6 and 24 h after infusion and samples of pancreatic tissue and plasma were obtained, immediately frozen and maintained at -80°C until assayed.
In a second series of experiments, a PPARγ agonist (Rosiglitazone, AVANDIA® GlaxoSmithKline, Brentford, UK) was administered (10 mg/kg intragastric bolus) 1 h before infusion of contrast medium (n = 8 for each group)[11]. Samples of plasma and pancreas were obtained 6 h after infusion.
The extent of pancreas edema was assayed by measuring tissue water content. Freshly obtained samples of pancreas were weighted on aluminum foil, dried for 24 h at 95°Cand reweighed. The difference between wet and dry tissue weight was calculated and expressed as a tissue wet: dry mass ratio.
Neutrophil infiltration was assessed by measuring myeloperoxidase (MPO) activity. Myeloperoxidase was measured photometrically with 3,3’,5,5’-tetramethylbenzidine as a substrate[16]. Samples were macerated with 5 g/L hexadecyltrimethylammonium bromide in 50 mmol/L phosphate buffer pH 6.0. Homogenates where then disrupted for 30 s using a Labsonic (B.Braun) sonicator at 20% power and submitted to three cycle of snap freezing in dry ice and thawing before a final 30 s sonication. Samples were incubated at 60°C for 2 h and then spun down at 4000 g for 12 min. Supernatants were collected for myeloperoxidase assay. Enzyme activity was assessed photometrically at 630 nm. The assay mixture consisted of 20 μL supernatant, 10 μL tetramethylbenzidine (final concentration 1.6 mmol/L) dissolved in DMSO and 70 μL H2O2 (final concentration 3.0 mmol/L) diluted in 80 mmol/L phosphate buffer pH 5.4. An enzyme unit is defined as the amount of enzyme that produces an increase of 1 absorbance unit per minute.
Binding of NFκB p65 subunit to the NFκB binding consensus sequence 5’-GGGACTTTCC-3’ and binding of PPARγ to the PPRE binding consensus sequence 5’-AACTAGGTCAAAGGTCA-3’ were measured with the ELISA-based TransAM kits (Active Motif, Carlsbad, CA) using tissue nuclear extracts. This assay is performed in 96-well plates coated with an oligonucleotide containing the binding consensus sequence. The active forms in nuclear extracts can be detected using specific Abs for epitopes that are accessible only when the subunits are activated and bound to its target DNA. Specificities were checked by measuring the ability of soluble wild-type oligonucleotides to inhibit binding.
Pancreatic tissue was lysed by using the Nuclear Extract Kit from Active Motif (Carlsbad, CA) following the manufacturer conditions for preparation of cytoplasmatic and nuclear extracts. SDS-PAGE was performed using 100 g/L or 120 g/L acrylamide gels. Proteins were electrotransfered to nitrocellulose membrane and probed with primary Ab (anti-p65, 1/1000; anti-PPARγ, 1/200; anti-iNOS, 1/400; anti-β-actin, 1/400; anti-Histone H1, 1/500). The membranes were incubated with corresponding horseradish peroxidase-linked secondary Ab, washed and subsequently incubated with ECL reagents from Amersham Pharmacia (Buckinghamshire, England) before exposure to high performance chemiluminescence films. Gels were calibrated using Bio-Rad standard proteins (Hercules, CA) with markers covering a 7-240 kDa range.
Total protein concentration in homogenates was deter-mined using a commercial kit from BioRad (Munich, Germany).
Plasma lipase was determined by using commercial kits from Randox (Antrim, UK), according to the supplier’s specifications.
Pancreatic tissue samples were taken and fixed in 40 g/L neutral buffered formaldehyde solution, paraplast-embedded, cut into 5 μm sections and stained with hematoxilyn-eosin for light microscopy. Two different observers evaluated randomly ten fields from each animal and cell infiltration was recorded blindly on photomicrographs.
Data have been expressed as mean ± SE. Means of different groups were compared using a one-way analysis of variance. Tukey’s multiple comparison test was performed for evaluation of significant differences between groups. Differences were assumed to be significant when P < 0.05.
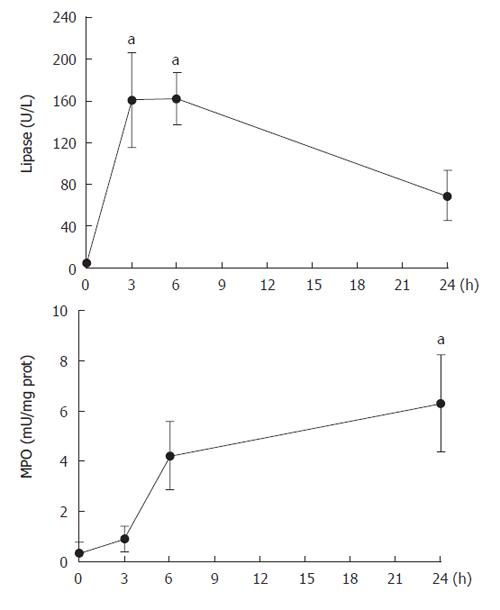
The evolution of pancreatic damage was evaluated by measuring plasma lipase and tissue MPO activity at different time points after pancreas infusion of contrast medium (Figure 1). A rapid and significant increase was observed in plasma lipase activity that achieved a peak between 3 and 6 h after surgery. By contrast, MPO activity was not increased until 6 h after infusion and remained increased until the end of experiment (24 h). Since in this model the inflammation required 6 h to be established, we selected this time point for the rest of experiments.
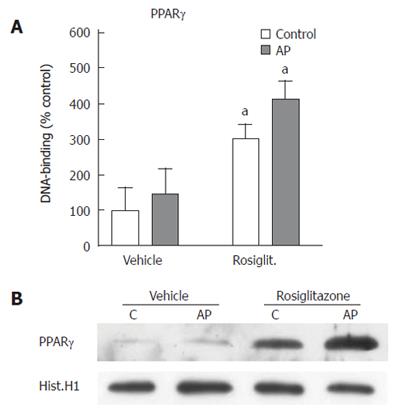
To evaluate the efficacy of rosiglitazone to induce PPARγ activation in the pancreatic tissue, we measured, on nuclear extracts, the levels of DNA binding activity to an immobilized oligonucleotide containing the PPRE sequence. Binding activity was significantly increased in rosiglitazone-treated animals (Figure 2A). By contrast, in non-treated animals, ERCP-induced pancreatitis was not associated with changes in pancreatic PPARγ activity. These results were confirmed by western blot analysis of the translocation of PPARγ into the nuclear fraction upon rosiglitazone treatment (Figure 2B).
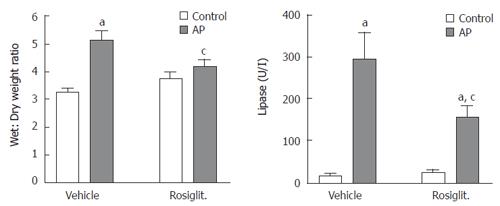
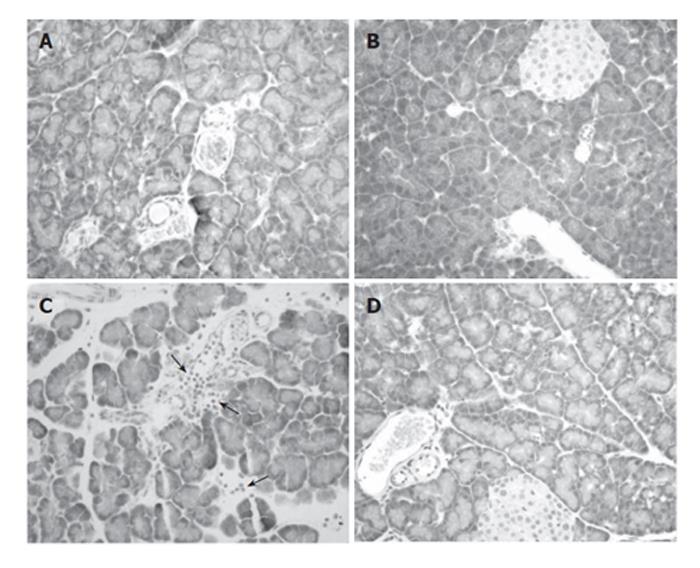
Both tissue edema and plasma lipase activity showed increased levels 6 h after contrast media infusion (Figure 3). Pre-treatment with rosiglitazone significantly reduced these increases, but not to control levels. Histological findings also showed a clear reduction on pancreatic interlobular edema (Figure 4). No acinar necrosis was observed after contrast-media infusion.
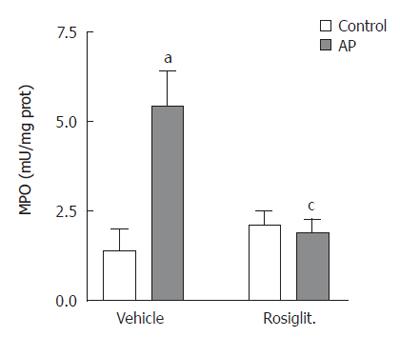
When measuring the MPO activity, we observed that the increase induced by contrast medium infusion was completely abrogated by pre-treatment with rosiglitazone (Figure 5). This result was confirmed by histological results. In ERCP group, areas of intense cell infiltration with extravasation of leukocytes to the interacinar space were observed (Figure 4C). Treatment with rosiglitazone completely prevented the infiltration of leukocytes (Figure 4D).
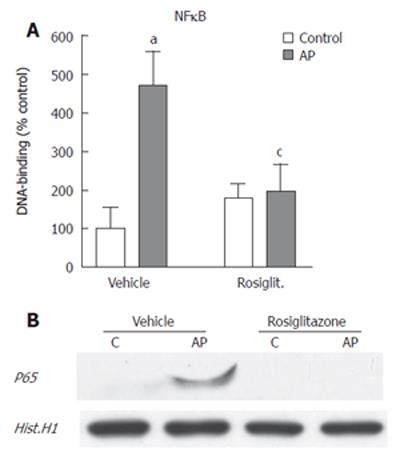
In order to evaluate the possible involvement of NFκB on this anti-inflammatory effect, we measured the levels of p65 DNA binding activity in pancreatic nuclear extracts. Results indicated that infusion of contrast media into the pancreatic duct induced a significant activation of NFκB (Figure 6A). This increase was completely prevented by pre-treatment of rosiglitazone. The effect of the PPAR-γ agonist on NFκB was confirmed by detecting, by western blot, the presence of p65 subunit of NFκB into the nuclear fraction (Figure 6B). In the ERCP group, p65 translocated into the nucleus and this translocation was prevented by pre-treatment of rosiglitazone.
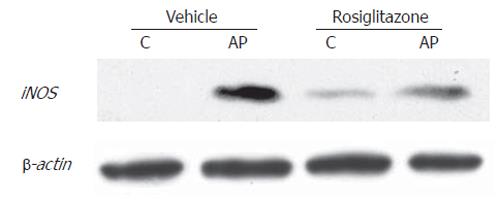
Finally, similar result was found when determining the expression of iNOS, an NFκB-dependent enzyme (Figure 7). In ERCP group, iNOS was strongly induced, and pre-treatment with rosiglitazone prevented this increase. Interestingly, the enzyme, that is undetectable in control animals showed a weak expression in control animals treated with rosiglitazone.
Since pancreatitis represents the most common complication after ERCP, different drugs, including IL-10, gabexate mesylate, heparin or somatostatin, nitrate derivates or diclofenac have been tested to reduce the incidence and severity of post ERCP-pancreatitis. Many of these studies, despite the use of randomized procedures were criticized because of non reproducible results[3]. In addition, the multifactorial etiology and pathophysiology of post-ERCP pancreatitis needs to be taken into account. Simplified procedures and the absence of adverse effects are required to deliver prophylactic treatment for post-ERCP pancreatitis. Our study shows that administration of a PPARγ agonist in a single oral dose before starting ERCP decreases the severity of the inflammatory reaction triggered by this procedure. Evidence has been accumulated indicating that PPARγ plays a role modulating the inflammation. Several studies have demonstrated that the use of PPARγ ligands inhibits the intensity of the inflammatory response in different processes including colitis[17], adjuvant-induced arthritis[14], and cerulein-induced pancreatitis[11]. In vitro, the expression of inflammatory mediators such as TNFα, IL-1β, IL-6, iNOS or MMP-9[13,18] could be inhibited by PPARγ ligands. These findings have raised the possibility that these agents could be useful for the treatment of the inflammatory disorders.
Our results indicate that administration of rosiglitazone before experimental ERCP completely prevented the inflammatory response in the pancreas, reflected in a reduced MPO activity and the lack of leukocytes infiltrate observed in pancreas. By contrast, increases in lipase plasma activity and edema were only partially prevented by rosiglitazone. This fact was not unexpected, since rosiglitazone prevents the activation of the inflammatory response, but has no effect on the mechanical damage related with changes in osmolarity or increased intraductal pressure. Pancreatic damage results from both mechanical and inflammatory processes associated with intraductal activation of pancreatic proenzymes. Nitrate derivates or pancreatic stenting have been proposed to increase pancreatic out-flow, diclofenac or IL-10 following inflammatory response and somatostatin to decrease the intraductal concentration of pancreatic proenzymes[5-9]. Rosiglitazone was supposed to act only on the inflammatory-related increases in lipase and edema.
It is known that the activation of PPARγ results in a reduction of the inflammatory response due to its inhibitory effect on main inflammatory signal transduction pathways, in particular, NFκB. It has been reported, in several experimental models of pancreatitis, that pancreatic damage was associated with increased nuclear translocation of NFκB dimers p65/p50 that trigger the transcription and generation of a broad spectrum of inflammatory mediators by pancreatic cells[19]. These mediators, including cytokines, chemokines and adhesion molecules, generate a microenvironment that promotes the recruitment and activation of inflammatory cells and contributes to increasing the extension of the pancreatic damage. Consequently, we have evaluated the involvement of NFκB in the observed anti-inflammatory effect of PPARγ in post-ERCP pancreatitis. For this purpose we have measured the nuclear translocation and DNA binding of p65, the key component of NFκB. The results indicate that nuclear translocation of p65 was significantly increased after experimental ERCP and this increase was completely prevented by pre-treatment of rosiglitazone. This inhibition could explain the lack of inflammatory infiltrate observed in pancreas, since NFκB activation is a requisite for the generation of the main pro-inflammatory mediators involved in pancreatitis.
Similar results were observed when measuring the expression of iNOS in pancreatic tissue. This enzyme was induced mainly in activated leukocytes in order to generate nitric oxide at a cytotoxic concentration as a part of the bactericidal mechanism of these cells. The presence of iNOS in pancreas in post-ERCP pancreatitis confirms that an intense inflammatory process was triggered. Since the synthesis of this enzyme is strongly dependent on NFκB, it is not a surprise that rosiglitazone treatment downregulates the expression of iNOS. On the other hand, the reduced levels of iNOS could also reflect the lack of cell infiltration that occurs under these conditions.
The blockage of NFκB activation and the resulting inhibition in the NFκB-dependent mediators is of importance not only for the local inflammation, but also in order to prevent the release into the circulatory bloodstream of pro-inflammatory cytokines that could trigger a systemic inflammatory response. Although this occurs in a reduced percentage of patients, the severity of the process justifies the use of prophylactic measures to prevent it despite the failure or the weakness of many previous series.
In conclusion, despite the general limitations of all the animal models relative to clinical setting and the multifactorial etiology of post-ERCP pancreatitis, these results suggest that rosiglitazone and other PPARγ agonists are potential new therapeutic agents for the prevention of post-ERCP-induced acute pancreatitis. It could be administered in an oral dose to the patients, shortly before the ERCP. However, further studies are needed to determine the proper dose and time-point of administration in human patients.
We thank Dr. Martín Rios for his help in the statistical analysis.
S- Editor Pan BR L- Editor Ma JY E- Editor Liu WF
| 1. | Barthet M, Lesavre N, Desjeux A, Gasmi M, Berthezene P, Berdah S, Viviand X, Grimaud JC. Complications of endoscopic sphincterotomy: results from a single tertiary referral center. Endoscopy. 2002;34:991-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Bjorkman DJ, Overby CS, Aas J, Ryan ME, Bochna GS. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 835] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 3. | Andriulli A, Leandro G, Niro G, Mangia A, Festa V, Gambassi G, Villani MR, Facciorusso D, Conoscitore P, Spirito F. Pharmacologic treatment can prevent pancreatic injury after ERCP: a meta-analysis. Gastrointest Endosc. 2000;51:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 125] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Hackert T, Werner J, Gebhard MM, Klar E. Effects of heparin in experimental models of acute pancreatitis and post-ERCP pancreatitis. Surgery. 2004;135:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Deviere J, Le Moine O, Van Laethem JL, Eisendrath P, Ghilain A, Severs N, Cohard M. Interleukin 10 reduces the incidence of pancreatitis after therapeutic endoscopic retrograde cholangiopancreatography. Gastroenterology. 2001;120:498-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Arvanitidis D, Anagnostopoulos GK, Giannopoulos D, Pantes A, Agaritsi R, Margantinis G, Tsiakos S, Sakorafas G, Kostopoulos P. Can somatostatin prevent post-ERCP pancreatitis? Results of a randomized controlled trial. J Gastroenterol Hepatol. 2004;19:278-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Moretó M, Zaballa M, Casado I, Merino O, Rueda M, Ramírez K, Urcelay R, Baranda A. Transdermal glyceryl trinitrate for prevention of post-ERCP pancreatitis: A randomized double-blind trial. Gastrointest Endosc. 2003;57:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Murray B, Carter R, Imrie C, Evans S, O’Suilleabhain C. Diclofenac reduces the incidence of acute pancreatitis after endoscopic retrograde cholangiopancreatography. Gastroenterology. 2003;124:1786-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Freeman ML, Guda NM. Prevention of post-ERCP pancreatitis: a comprehensive review. Gastrointest Endosc. 2004;59:845-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 316] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 10. | Clark RB. The role of PPARs in inflammation and immunity. J Leukoc Biol. 2002;71:388-400. [PubMed] |
| 11. | Cuzzocrea S, Pisano B, Dugo L, Ianaro A, Britti D, Patel NS, Di Paola R, Genovese T, Di Rosa M, Caputi AP. Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-gamma, reduces acute pancreatitis induced by cerulein. Intensive Care Med. 2004;30:951-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Nakajima A, Wada K, Miki H, Kubota N, Nakajima N, Terauchi Y, Ohnishi S, Saubermann LJ, Kadowaki T, Blumberg RS. Endogenous PPAR gamma mediates anti-inflammatory activity in murine ischemia-reperfusion injury. Gastroenterology. 2001;120:460-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 181] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2807] [Cited by in RCA: 2816] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 14. | Shiojiri T, Wada K, Nakajima A, Katayama K, Shibuya A, Kudo C, Kadowaki T, Mayumi T, Yura Y, Kamisaki Y. PPAR gamma ligands inhibit nitrotyrosine formation and inflammatory mediator expressions in adjuvant-induced rheumatoid arthritis mice. Eur J Pharmacol. 2002;448:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | He ZJ, Winston JH, Yusuf TE, Micci MA, Elfert A, Xiao SY, Pasricha PJ. Intraductal administration of an NK1 receptor antagonist attenuates the inflammatory response to retrograde infusion of radiological contrast in rats: implications for the pathogenesis and prevention of ERCP-induced pancreatitis. Pancreas. 2003;27:e13-e17. [PubMed] |
| 16. | Trush MA, Egner PA, Kensler TW. Myeloperoxidase as a biomarker of skin irritation and inflammation. Food Chem Toxicol. 1994;32:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Sánchez-Hidalgo M, Martín AR, Villegas I, Alarcón De La Lastra C. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor gamma, reduces chronic colonic inflammation in rats. Biochem Pharmacol. 2005;69:1733-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Zafiriou S, Stanners SR, Saad S, Polhill TS, Poronnik P, Pollock CA. Pioglitazone inhibits cell growth and reduces matrix production in human kidney fibroblasts. J Am Soc Nephrol. 2005;16:638-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Vaquero E, Gukovsky I, Zaninovic V, Gukovskaya AS, Pandol SJ. Localized pancreatic NF-kappaB activation and inflammatory response in taurocholate-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1197-G1208. [PubMed] |