INTRODUCTION
Colorectal cancer (CRC) is the second most lethal cancer in the USA. Numerous studies suggest that nonsteroidal anti-inflammatory drugs (NSAIDs) have antineoplastic effects. Epidemiological studies have shown a reduction in the risk of death from colorectal cancer associated with the use of NSAIDs[1]. Perhaps the most compelling evidence for the chemopreventive role of NSAIDs is provided by the clinical studies in patients with familial adenomatous polyposis (FAP), an inherited predisposition for CRC[2-6]. In these studies, the NSAID sulindac and the selective cyclooxygenase-2 inhibitor (COX-2) celecoxib, reduced both the number and the size of colorectal adenomas in FAP patients. Similarly, NSAIDs prevent tumor formation in a variety of animal models of CRC[7,8].
How exactly do NSAIDs exert their effects to prevent tumorigenesis? A large body of evidence suggests that NSAIDs exert their chemopreventive actions by: (1) inhibition of cell growth and proliferation and (2) induction of apoptosis[9]. The mechanisms by which NSAIDs achieve these processes are still incompletely understood. One widely studied mechanism is via the COX-2 dependent pathways.
NSAIDs are known as the inhibitors of the cyclooxygenase (COX-1 and COX-2) enzymes, which are involved in the conversion of arachidonic acid to eicosanoids. COX-1 is expressed constitutively in a variety of tissues and plays a role in platelet aggregation and gastric cytoprotection. COX-2, is inducible particularly during inflammation in cells such as endothelial cells, macrophages and intestinal epithelial cells[10]. Interestingly, COX-2 expression has been shown to be dramatically increased in colon cancer cells compared to normal colonic mucosa and is associated with a several-fold increase in concentration of prostaglandin E2 (PGE2)[11-13]. Studies from our lab demonstrated that COX-2 generated PGE2 promotes colon cancer cell growth by transactivating epidermal growth factor receptor[14]. The increased expression of COX-2 also seems to prevent cancer cells from undergoing programmed cell death, at the same time, promoting angiogenesis, enhancing metastatic potential and modulating cell proliferation. Direct evidence for the role of COX-2 in colon cancer tumorigenesis has been reported in many studies. Using APC knockout mice, which develop polyps in their intestinal tracts due to the mutation of the APC gene, Oshima and colleagues were able to show a significant reduction of intestinal polyps when either one or both of COX-2 alleles were deleted[15,16]. Furthermore, the COX-2 selective inhibitor NS-398, has been reported to suppress tumor growth in different cancer cell lines, to induce apoptosis in human colon cancer cells and to have anti-angiogenic effects[9,17-19].
However, recent data suggest that COX-2 may not be the sole target for the action of NSAIDs. The anti-neoplastic effects of NSAIDs are also seen in tumor cells that do not express COX[20]. Similarly, NSAID derivatives such as sulindac sulfone, which lacks the ability to inhibit COX, is shown to inhibit colon cancer growth[21]. Furthermore, NSAIDs affect other mechanisms that regulate cell proliferation and death. For example, NSAIDs regulate PPARδ, the NFκB pathway and the lipoxygenase pathway, all of which play a critical role in the regulation of cell survival and death[22-24].
In order to better understand the complex mechanisms underlying the cancer chemopreventive properties of NSAIDs, it is important to understand the signaling pathways and the genes that directly lead to the inhibition of proliferation and/or the induction of apoptosis in colon cancer cells. With the recent development of DNA microarray technology, identification of genes and pathways can be easily achieved by using microarray chips that contain the genes of interest.
This study was aimed to provide in depth insight into the pro-apoptotic actions of Indomethacin (IND), a nonselective COX inhibitor and NS-398 (NS), a COX-2 selective inhibitor on colon cancer Caco-2 cells, and identify specific pathways and genes involved.
MATERIALS AND METHODS
Cell culture
Human colon cancer (Caco-2) cells (ATCC, Rockville, MD, USA) were grown in DMEM/F12 medium supplied with 20% fetal bovine serum and antibiotics at 37°C in a humidified incubator containing 50 mL/L of CO2 .
Treatment of cells with Indomethacin and NS-398
A 100 mmol/L stock solution IND (Sigma Chemical, St. Louis, MO, USA) was freshly prepared by dissolving the compound in 0.2 mol/L Na2CO3 and 1 mol/L NaH2PO4, and kept at 37°C before treatment. A 50 mmol/L stock solution of NS (Cayman Chemical, Ann Arbor, MI) was prepared by dissolving the compound in dimethyl sulfoxide. Caco-2 cells were plated in dishes (100 mm × 20 mm) at a density of 1 × 106 and kept in serum-free medium for 24 h before treatment. The cells were subsequently treated with IND (0.05 mmol/L, 0.1 mmol/L and 0.5 mmol/L) or NS (0.01 mmol/L, 0.1 mmol/L and 0.2 mmol/L) for 1, 5 and 18 h at 37°C.
Cell death detection assay
Cells were plated in 8-well chamber slides and grown until 50%-75% confluent. They were subsequently treated with IND and NS in the same way as described above. Cell death was evaluated by terminal deoxynucleotide transferase (TdT)-mediated dUTP nick end-labeling (TUNEL) using a commercial kit (Roche Diagnostics, Indianapolis, IN, USA, Cat No. 1 684 817). Briefly, cell monolayers were air-dried and fixed with 4% paraformaldehyde for 1 h at room temperature. 3% H2O2 was then used to block endogenous peroxidase. The TUNEL reaction mixture was applied to the cells and incubated for 1 h. Following incubation with anti-fluorescein antibody conjugated with horseradish peroxidase (HRP), diaminobenzidine substrate was then used as developing the agent. Cells treated with culture medium without NSAIDs served as controls. Apoptosis was evaluated by counting the number of apoptotic and total cells in five random fields per chamber in each slide. Apoptotic index was expressed as percentage of apoptotic cells in total number of counted cells.
cDNA microarray study
Serum starved Caco-2 cells were treated with IND or NS in the same way as described above. Total RNA was isolated with Trizol reagent (Invitrogen, Carlsbad, CA, USA, Cat No. 15596-026). Equal amount of RNA from each treatment was used as templates for cDNA synthesis. Radiolabeled probes were synthesized using 32P dCTP following the manufacturer’s protocol provided with the microarray study kit (GEArray Q series, SuperArray Inc. Bethesda, MD, USA). The denatured cDNA probes were added to the arrays containing the genes to be studied. Each of the arrays contains 96 cDNA fragments from genes associated with a specific apoptotic pathway. After overnight hybridization at 60°C with continuous agitation, the arrays were washed for 4 × 15 min with each washing solution (2 × SSC, 1% SDS, then 0.1 × SSC, 0.5% SDS). The arrays were then developed using X-ray films. Quantification of the images was accomplished by the ImageQuant software (Molecular Dynamics, Sunnyvale, CA, USA). Results were expressed in fold change.
Western blotting
Serum starved Caco-2 cells were treated with IND or NS in the same way as described above. Total protein was isolated in a lysis buffer containing 20 mmol/L Tris-HCl pH 7.5, 1 mmol/L EDTA, 300 mmol/L NaCl, 0.2 mmol/L Sodium vanadate, 1% Triton x-100 and 1% NP-40. Equal amount of protein lysate from each treatment was separated by SDA-PAGE and then transferred electrophoretically to a nitrocellulose membrane. The blots were probed with the following antibodies: APAF1 (apoptotic protease activating factor), BAK (BCL2-antagonist/killer 1), BAX (BCL2-associated X protein), DFFA (DNA fragment factor-45), XIAP (X-linked IAP) and Survivin. The signal was visualized by the chemiluminescence method using ECL detection reagents (Amersham Life Sciences, Arlington Heights, IL). Signals were quantified by densitometry scanning.
Statistical analysis
Results from the TUNEL study are expressed as the mean ± the standard error. Student’s t test was used to determine statistical difference between controls and treatments. A P value of < 0.05 was considered statistically significant. Comparisons of data between multiple groups were performed with ANOVA.
RESULTS
Apoptotic cell death detection
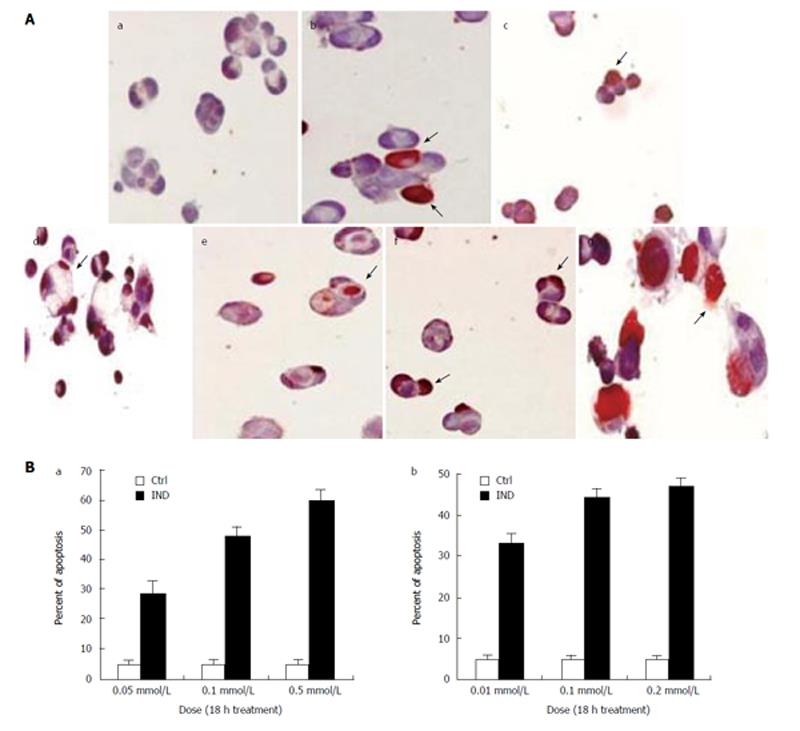
Using TUNEL method, we found a significant increase in apoptotic cell death in Caco-2 cells treated with both the nonselective COX inhibitor IND and the COX-2-selective inhibitor NS as compared to placebo. The IND and NS-induced apoptotic cell death was dose dependent (Figure 1A and B). Treatment with IND at 0.05 mmol/L, 0.1 mmol/L and 0.5 mmol/L resulted in 28% ± 5%, 48% ± 3% and 60% ± 4% of apoptosis respectively (all P < 0.001 vs control). On the other hand, treatment with NS at 0.01 mmol/L, 0.1 mmol/L and 0.2 mmol/L induced apoptosis in 33% ± 3%, 44% ± 2% and 47% ± 1% respectively (all P < 0.001 vs control). In addition, at 0.1mmol/L concentration, IND induced apoptosis in 33% ± 4%, 31% ± 2% and 48% ± 5% (all P < 0.001 vs control) of cells and NS induced apoptosis in 29% ± 3%, 26% ± 4% and 46% ± 6% (all P < 0.001 vs control) of cells at 1, 5 and 18 h, respectively (graph not shown).
Figure 1 A: TUNEL staining of Caco-2 cells.
IND and NS treatment cause apoptosis in a dose dependent manner after 18 h. (a) Control; (b) IND 0.05 mmol/L; (c) IND 0.1 mmol/L; (d) IND 0.5 mmol/L; (e) NS 0.01 mmol/L; (f) NS 0.1 mmol/L; (g) NS 0.2 mmol/L. Experiments performed in triplicates; B: Apoptosis in Caco-2 cells is dose dependent after treatment with (a) IND and (b) NS for 18 h. Experiments performed in triplicates.
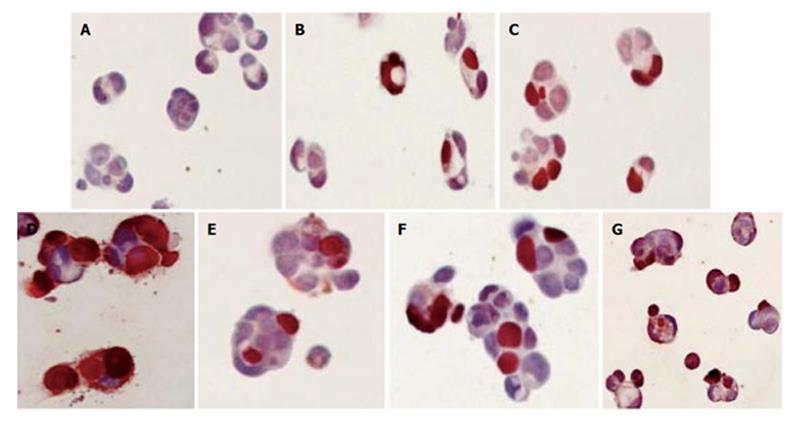
IND and NS treatment induced morphological changes consistent with apoptosis (cell shrinkage, condensation of chromatin, cytoplasmic budding, apoptotic bodies), which occurred as early as 1 h post-treatment and became extensively evident in 5 and 18 h (Figure 2).
Figure 2 TUNEL staining of Caco-2 cells treated with IND (0.
1 mmol/L) and NS (0.1 mmol/L) for 1, 5 and 18 h. (A) Control; (B) IND for 1 h; (C) IND for 5 h; (D) IND for 18 h; (E) NS for 1 h; (F) NS for 5 h; (G) NS for 18 h.
cDNA microarray study of 96 apoptosis related genes
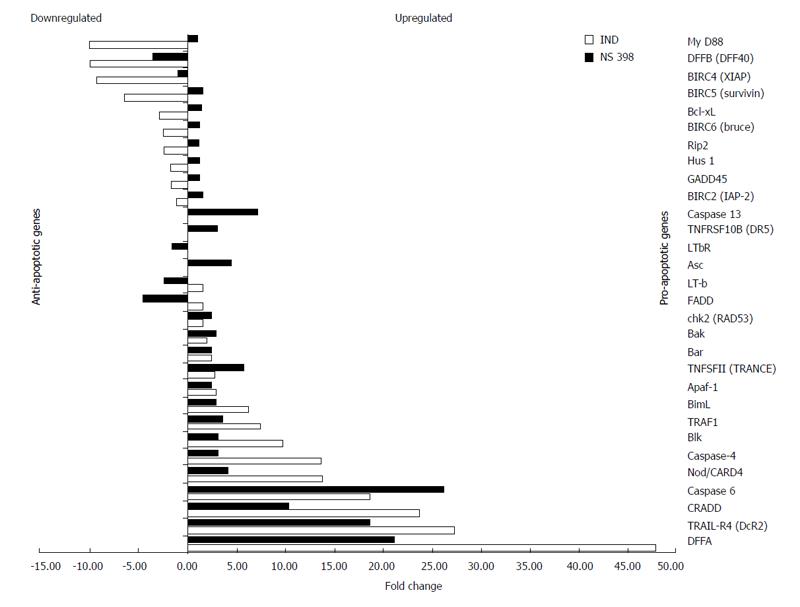
IND treatment (0.1 mmol/L for 1 h) caused a significant up-regulation of pro-apoptotic genes in mainly four families: (I) TNF receptor and ligand (TRAIL-R4-A by 27-fold, TNFSF11 by 3-fold); (II) Caspase (CASP4 by 14-fold, CASP6 by 19-fold); (III) BCL-2 (BCL2L11/bimL by 6-fold, Blk by 10-fold); (IV) Caspase recruiting domain (Apaf-1 by 3-fold, NOD/CARD4 by 14-fold).
In addition, IND significantly up-regulated other genes such as DNA fragmentation factor 45/DFFA (by 48-fold) and CRADD (by 24-fold). In addition to the genes up-regulated by IND, NS up-regulated some other genes within these families such as CASP13 (7-fold), Asc (5-fold), TRAIL receptor 2 (3-fold) and Bak (3-fold). The transcriptional up-regulation of these pro-apoptotic genes occurred as early as 1 h after drug exposure. We also found that IND, but not NS, down-regulated most of the anti-apoptotic genes in the IAP (inhibitor of apoptosis protein) family such as IAP2, XIAP (9-fold), survivin (7-fold) and Bruce (3-fold) (Figure 3). Significant up-regulation and down-regulation of apoptotic-related genes in our study is determined by a 3 or more fold change from control.
Figure 3 Genes up-regulated and down-regulated by IND and NS treatment identified by cDNA microarray.
Up-regulation or down-regulation is expressed as a fold change. Down-regulated genes are shown on the top of the graph in a decreasing order toward mid graph, while up-regulated genes in an increasing order toward the bottom of the graph. Anti-apoptotic genes lie mostly in the upper half while pro-apoptotic genes lie mainly in the bottom half.
Protein analysis
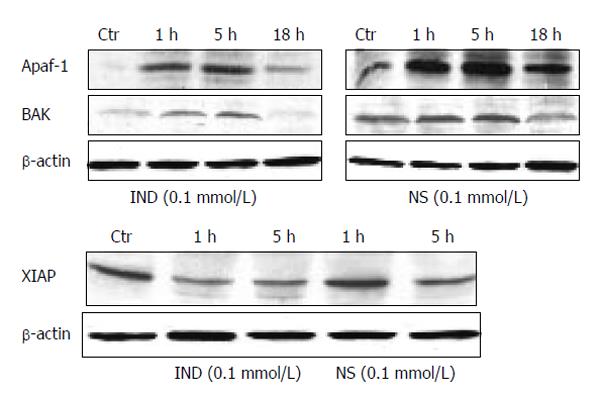
The protein expression of several significantly up-regulated or down-regulated genes is shown in Figure 4. Both IND and NS treatments up-regulated the pro-apoptotic protein Apaf-1 significantly by 9, 12, and 4 fold at 1, 5, and 18 h, respectively (IND); by 96%, 160% and 102% at 1, 5 and 18 h, respectively (NS) (all P < 0.01 vs control). In addition, both of these drugs also increased Bak protein expression. However, IND treatment also down-regulated anti-apoptotic proteins such as XIAP by 40% and 22% at 1 and 5 h respectively (P < 0.05 vs control), while NS treatment did not alter it significantly.
Figure 4 Protein expression by Western blotting of pro-apoptotic genes Apaf-1 and Bak (upper panel), expression of XIAP (lower panel).
Blots are representative of triplicate experiments.
DISCUSSION
The cure for colorectal cancer remains a challenge despite all currently available medical and surgical treatment modalities. The cure rate is particularly low in advanced disease, making chemoprevention an attractive alternative. In recent years, the NSAIDs have attracted a great interest as a potential candidate for the chemoprevention of colorectal cancer. There is now a great body of evidence supporting the role of NSAIDs in tumor chemoprevention by the induction of apoptosis. However, little is known about the exact mechanisms of NSAIDs action on colorectal cancer cells.
With the advent of microarray technology, thousands of genes can be assayed in a single experiment. We applied the microarray technology and focused on apoptosis related genes. By creating a profile of genes linked to a particular biological pathway, the expression of particular genes in a colon cancer cell line treated with NSAIDs, may suggest the activation of specific pathways. Furthermore, comparing the action of a nonselective COX inhibitor vs a COX-2-selective inhibitor can reveal mechanistic differences between these two NSAIDs in the induction of apoptotic cell death.
Our results suggest that both the nonselective COX inhibitor indomethacin and the COX-2-selective inhibitor NS-398 trigger apoptosis in Caco-2 cells through at least two major well known pathways, an extrinsic, death receptor-transmembrane pathway and an intrinsic, mitochondrial pathway.
The transmembrane pathway of apoptosis involves the tumor necrosis factor (TNF) ligand and receptor superfamily. Members of this family include TNFα, Fas ligand and TRAIL (TNF-related apoptosis-inducing ligand). These ligands when coupling to their respective receptors trigger a number of intracellular events that lead to apoptotic cell death[25,26]. The final steps of apoptosis induced through this pathway depends on the activation of caspases, cysteine proteases that cleave the aspartate residues[27,28]. Several members of the TNF family of receptors contain intracellular death domains that interact with other molecules that contain death domains and with caspase recruitment domains (CARD), propagating the apoptotic cascade[29,30]. We found that both IND and NS-398 up-regulated several genes within the TNF ligand and receptor family such as TNFSF11 (Tumor necrosis factor superfamily member 11), TRAIL-R4, TRAIL-R2. We also detected up-regulation of other genes encoding downstream molecules associated with this pathway such as CRADD (CASP2 and RIPK1 domain containing adaptor with death domain), ASC (Apoptosis-associated speck-like protein containing a CARD), NOD1 (caspase recruitment domain 4), APAF (apoptotic protease activating factor). Our data strongly suggest that NSAIDs induce apoptosis in Caco-2 cells by up-regulating genes of the TNF ligand and receptor pathway, likely mediated by TNF receptors and TRAIL receptors. This mechanism may explain in part the chemopreventive properties of NSAIDs in colorectal cancer. Several studies have shown promise in using TNFα and TRAIL as potential chemotherapeutic agents in a variety of tumors[31-34]; however, toxicity remains a concern when these agents are used systemically. Our study provides further evidence that NSAIDs likely sensitize colorectal cells to death through TNF and TRAIL receptor. This suggests NSAIDs to be potentially useful as an adjunct chemotherapeutic agent by increasing the therapeutic window of TNFα and TRAIL and decreasing their toxicity.
Our study suggests that NSAIDs also affect the mitochondrial pathway of apoptosis. This intrinsic pathway is usually triggered by chemicals, growth factor deprivation or irradiation. The major steps in this pathway involve the release of cytochrome c with subsequent increase in mitochondrial membrane permeability that is controlled by a variety of pro-apoptotic and anti-apoptotic members of the Bcl-2 family. The release of cytochrome c triggers the assembly of Apaf-1 (Apoptotic protease-activating factor) and pro-caspase 9 to form an apoptosome. The activated caspase 9 then activates other downstream molecules to initiate apoptosis[35-37]. Several members of the Bcl-2 family were shown to be up-regulated or down-regulated in Caco-2 cells treated with IND and NS. The pro-apoptotic members up-regulated by NSAIDs include Bak (Bcl2-antagonist/killer 1), Blk (B lymphoid thyrosine kinase) and Bim (Bcl2 like 11, apoptosis facilitator). Bcl-x (Bcl2-like 1), an antiapoptotic member, was down-regulated. These data suggest that NSAIDs, both COX nonselective and COX-2 selective likely induce apoptosis via the death receptor pathways and the mitochondrial pathway.
There was one major difference in the pattern of genes expressed in Caco-2 cells treated with IND and NS-398. While both IND and NS-398 up-regulated pro-apoptotic genes, IND also down-regulated anti-apoptotic genes, particularly the IAP (inhibitors of apoptosis proteins) family. This family has been described consisting of at least six members and can efficiently inhibit the caspases[38-40]. In our study, IND treatment of Caco-2 cells caused significantly down-regulated IAP2, XIAP, Survivin and Bruce, while treatment with NS-398 did not affect these genes. This may suggest a mechanistic difference by which non-selective COX and COX-2 specific NSAIDs induce apoptosis. This finding indicates that COX-1 may play a role in the different genetic expression of the IAP family between IND and NS treatments. Further functional studies exploring this difference may contribute to a better understanding of the chemopreventive properties of NSAIDs.