INTRODUCTION
The differentiation of autoimmune pancreatitis (AIP) from pancreatic cancer is sometimes difficult because these diseases share many clinical features[1]. When pancreatic cancer cannot be ruled out, laparotomy or pancreatic resection may be performed for patients with AIP. To date, there has been little information on long-term outcomes of patients with AIP treated with surgery. Some reports have described recurrences of AIP and pancreatic insufficiency after surgery[2-4]. However, there have been only two reports describing infections[4,5]. To our knowledge, this is the second reported case of liver abscess after surgical treatment of AIP.
CASE REPORT
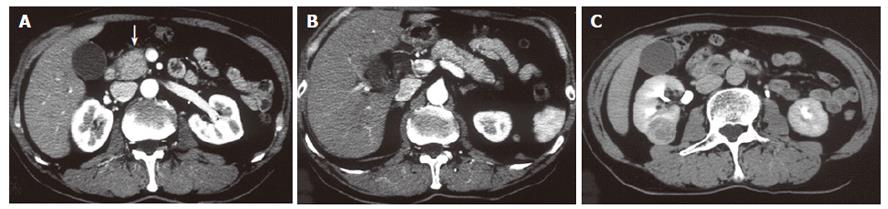
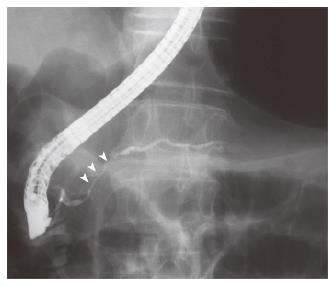
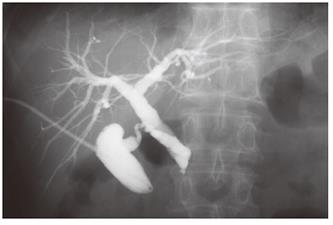
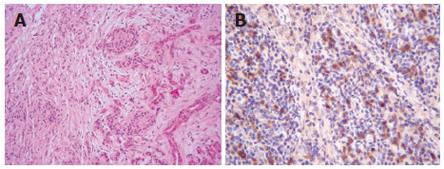
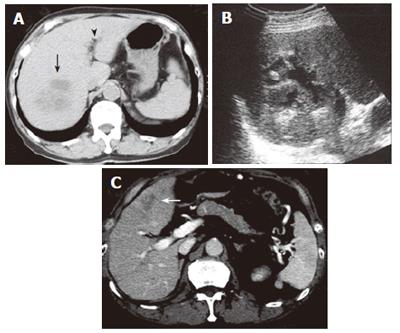
A 68-year-old man with bronchial asthma visited a neighborhood clinic. The patient began to feel abdominal fullness and decreased appetite in January 2000. After laboratory studies showed liver dysfunction and hyperglycemia, he was referred to our hospital in February 2000. He had no history of malignancy or autoimmune diseases. He was not a drinker. Physical examination showed no abnormal findings. Laboratory studies showed elevated liver enzymes and hyperbilirubinemia: total bilirubin was 34 mg/L (normal, 3-11) and direct bilirubin was 24 mg/L (normal, 0-6). Serum levels of pancreatic enzymes were as follows: amylase, 75 IU/L (normal, 50-200); lipase, 52 IU/L (normal, 8-46); and elastase I, 3900 ng/L (normal, 810-2960). A fasting plasma glucose test and an oral glucose tolerance test indicated diabetes mellitus. Serum levels of gammaglobulin (13.4 g/L) and IgG (14550 mg/L) were not elevated. Serum IgG4 was not examined. Antinuclear antibodies were negative. Serum levels of tumor markers, such as carcinoembryonic antigen and carbohydrate antigen 19-9, were normal. A dynamic computed tomography scan showed swelling of the gallbladder, dilatation of the common bile duct, and slight swelling of the pancreas head (3.5 cm × 3.0 cm) (Figure 1A and 1B). Furthermore, a 2.8-cm-diameter tumor was detected incidentally in the right kidney (Figure 1C). Endoscopic retrograde cholangiopancreatography showed that the main pancreatic duct in the head was irregularly narrowed and that the upstream duct was slightly dilated (Figure 2). Contrast agent could not be injected into the common bile duct. Percutaneous transhepatic gallbladder drainage was performed for obstructive jaundice. Fluorography through the biliary drainage catheter showed obstruction in the distal common bile duct (Figure 3). After biliary drainage, the patient underwent laparotomy in March 2000, because pancreatic cancer could not be ruled out and because a malignant renal tumor was suspected. Because wedge biopsy of the pancreas head showed no malignancy, cholecystectomy and choledochoduodenostomy were performed for biliary obstruction. Simultaneously, nephrectomy was performed for the renal tumor. Further histopathologic examination of the pancreas specimen revealed lymphoplasmacytic infiltration and interstitial fibrosis with acinar atrophy (Figure 4A). An immunohistologic study performed 5 years after surgery showed dense IgG4-positive plasma cell infiltration in peripancreatic lymph nodes (Figure 4B). The renal tumor was diagnosed as a renal cell cancer (clear cell carcinoma, stage I).
Figure 1 Dynamic computed tomography scan showing swelling of the gallbladder and slight swelling of the pancreas head (white arrow) (A).
The pancreas body and tail are not swollen (B). A tumor is detected in the right kidney (C).
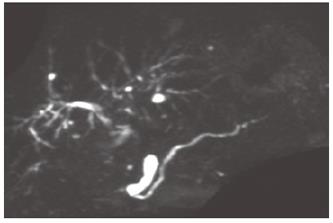
Figure 2 Endoscopic retrograde cholangiopancreatography showing short irregular narrowing of the main pancreatic duct in the head.
The narrowing is less than one third of the length of the main pancreatic duct (white arrowheads).
Figure 3 Percutaneous transhepatic gallbladder drainage.
Fluorography through the biliary drainage catheter shows obstruction of the distal common bile duct.
Figure 4 Wedge biopsy of the pancreas head reveals lymphoplasmacytic infiltration and interstitial fibrosis with acinar atrophy (HE x 100) (A).
Immunohistologic study shows dense IgG4-positive plasma cell infiltration in peripancreatic lymph nodes (HE x 200) (B).
Seven months after surgery, the patient complained of abdominal discomfort. Serum levels of pancreatic enzymes were markedly elevated: amylase, 621 IU/L; and lipase, 1082 IU/L. A serum level of carbohydrate antigen 19-9 was also elevated to 159.9 IU/mL (normal, 0-37). Dynamic computed tomography showed that swelling of the pancreas progressed from the head to the entire pancreas (Figure 5A and 5B). On the basis of clinical and histopathologic findings, AIP was diagnosed. Treatment with prednisolone of 5 mg per day was started. Thereafter, the symptom disappeared and serum levels of pancreatic enzymes were normalized. The swelling of the pancreas and irregular narrowing of the main pancreatic duct improved (Figure 6). Diabetes also improved.
Figure 5 Dynamic computed tomography scan showing swelling of the pancreas head.
A capsule-like low-density rim can be seen around the pancreas head (white arrowheads) (A). The pancreas body and tail are swollen (B).
Figure 6 Magnetic resonance cholangiopancreatography performed 4 years after steroid therapy.
The narrowing site is resolved in the main pancreatic duct.
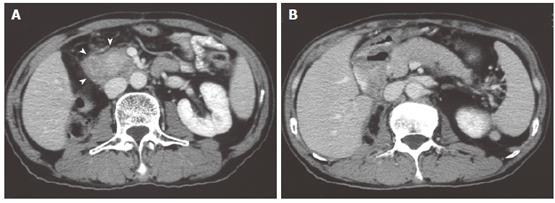
In February 2005, 5 years after surgery, laboratory studies showed slightly elevated biliary enzymes, but the patient had no symptoms. In April 2005, the patient was admitted because of acute pyrexia in a preshock state. At that time, he had been continuing to take oral prednisolone, 5 mg per day. Laboratory studies showed elevated liver enzymes and an inflammatory response: white blood cell count, 18.9 ×109/L (normal, 3.1-9.2 × 109/L) and C-reactive protein, 288 mg/L (normal, < 5). Blood culture was positive for Klebsiella pneumoniae. Ultrasonography and computed tomography scan showed a multilocular liver abscess, 5.6 cm × 5.3 cm in size, in the right anteroinferior segment (Figure 7A and 7B). Vasopressor was administered for the preshock state, and percutaneous abscess drainage was performed for the abscess in combination with intravenous administration of cefoperazone/sulbactam at a dosage of 2 g per day. Prednisolone was stopped. A pus culture also showed Klebsiella pneumoniae. The patient was discharged after 2 wk of treatment; however, a liver abscess occurred in the left medial segment 1 mo later (Figure 7C), but was successfully treated with antibiotics. Hepatobiliary scintigraphy suggested no decrease of bile flow. The patient has been in good health since the second liver abscess was treated. AIP has not recurred irrespective of cessation of steroid therapy.
Figure 7 Computed tomography scan showing an abscess in the right anteroinferior segment of the liver (black arrow).
Pneumobilia is detected (black arrowhead) (A). Ultrasonography shows partial liquefaction in the abscess (B). Computed tomography scan showing an abscess in the left medial segment of the liver (white arrow) (C). Note that the pancreas is not swollen.
DISCUSSION
The clinical entity of AIP was established when Yoshida et al[6] proposed the concept in 1995; however, a worldwide consensus on diagnostic criteria for AIP has not been achieved[7]. Retrospectively, the present case did not satisfy the diagnostic criteria proposed by the Japan Pancreas Society[8] at the onset of AIP. However, the results of histopathologic examinations[9] and the clinical findings after surgery were compatible with AIP. Recent reports have described various AIP cases, such as the present one, in which pathologic changes are not found throughout the pancreas[5,10-12]. These “focal” and “segmental” types of AIP have been considered to correspond to an early stage of AIP[10-12]. To avoid unnecessary laparotomy or pancreatic resection, the establishment of definitive diagnostic criteria for AIP is urgent. If AIP is suspected but pancreatic cancer cannot be ruled out by imaging and laboratory studies, endoscopic ultrasonography-guided trucut biopsy may help establish a diagnosis of AIP[13]. A short course of steroids can also be used to guide diagnosis[7].
Horiuchi et al[5] reported the first case of AIP associated with liver abscess. This is the second one. It should be noted that liver abscess developed several years after surgical treatment of AIP in both cases. The pathogenesis of liver abscess in the present case remains unclear. The route of infection seemed to be the biliary tract because other routes, such as the portal tract, were unlikely. AIP is often associated with sclerosing cholangitis[14-16]. Biliary stenosis caused by sclerosing cholangitis or anastomotic stenosis can induce cholestasis, which may cause biliary infection and liver abscess. However, cholestasis was not observed in the present case, suggesting that cholestasis is not a prerequisite for liver abscess formation after surgery. Furthermore, there have been few reports describing cases of liver abscess after choledochoduodenostomy. The sump syndrome, a complication of side-to-side choledochoduodenostomy, can cause secondary liver abscess[17]; however, the present patient had undergone end-to-side choledochoduodenostomy before the development of liver abscess. There may be other factors contributing to the development of the patient’s liver abscess in addition to duodeno-biliary reflux due to biliary reconstruction.
Putative predisposing factors for infection in patients with AIP are as follows. First, diabetes mellitus, which is often associated with AIP[18], can compromise patients’ defenses against infection. Recent reports have shown that diabetes is present in many patients with pyogenic liver abscess[19,20]. Second, steroid therapy for AIP can impair immunity. In most patients with AIP and diabetes, steroid therapy improves diabetes; however, in some cases, steroid therapy induces or exacerbates diabetes[18]. The present patient had been receiving long-term steroid therapy, which improved the diabetes but might have induced an immunocompromised state.
In conclusion, pyogenic liver abscess can occur as a late complication of biliary reconstruction in patients with AIP. Careful follow-up should be performed to allow early detection of such a serious complication in patients with AIP treated with surgery.