Published online Oct 14, 2006. doi: 10.3748/wjg.v12.i38.6219
Revised: July 28, 2006
Accepted: August 3, 2006
Published online: October 14, 2006
Metastatic breast cancer involving the hepatobiliary tract or ascites secondary to peritoneal carcinomatosis has been well described. Luminal gastrointestinal tract involvement is less common and recognition of the range of possible presentations is important for early and accurate diagnosis and treatment. We report 6 patients with a variety of presentations of metastatic breast cancer of the luminal gastrointestinal tract. These include oropharyngeal and esophageal involvement presenting as dysphagia with one case of pseudoachalasia, a linitis plastica-like picture with gastric narrowing and thickened folds, small bowel obstruction and multiple strictures mimicking Crohn’s disease, and a colonic neoplasm presenting with obstruction. Lobular carcinoma, representing only 10% of breast cancers is more likely to metastasize to the gastrointestinal tract. These patients presented with gastrointestinal manifestations after an average of 9.5 years and as long as 20 years from initial diagnosis of breast cancer. Given the increased survival of breast cancer patients with current therapeutic regimes, more unusual presentations of metastatic disease, including involvement of the gastrointestinal tract can be anticipated.
- Citation: Nazareno J, Taves D, Preiksaitis HG. Metastatic breast cancer to the gastrointestinal tract: A case series and review of the literature. World J Gastroenterol 2006; 12(38): 6219-6224
- URL: https://www.wjgnet.com/1007-9327/full/v12/i38/6219.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i38.6219
Breast cancer is the most common neoplasm in women, accounting for approximately 32% of cancers in women, with a lifetime risk of 1 in 10[1]. Prognosis is related to the presence of hormonal receptors, the size and grade of the primary tumour, the presence of regional lymphadenopathy, and metastatic disease[1]. Metastatic breast cancer typically involves the lungs, bones, brain and liver, but occasionally can affect the gastrointestinal tract[2]. We report 6 patients with a variety of presentations involving the gastrointestinal tract, all of which were eventually found to be due to metastatic breast cancer. These examples highlight the importance of considering metastatic breast cancer as a potential cause of radiological abnormalities affecting any part of the gastrointestinal tract of women, especially those with a previous history of breast cancer.
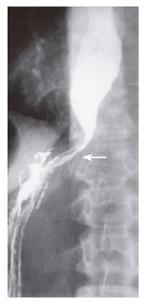
A 51-year old female presented with a 3-mo history of progressive solid food dysphagia. Initial barium swallow and endoscopy were normal. Esophageal manometry showed impaired relaxation of the lower esophageal sphincter, but no other abnormality. A decision was made to follow the patient and re-investigate her in 6 mo. The patient returned after 5 mo with worsening dysphagia and 20-pound weight loss. Barium swallow demonstrated retained secretions, esophageal dilatation and tapered narrowing in the region of the lower esophageal sphincter (Figure 1). Repeat manometry demonstrated changes consistent with achalasia. Repeat endoscopy showed a dilated esophagus and a narrowed gastroesophageal junction with no intrinsic mucosal abnormality. Pneumatic dilatation with a Rigiflex 35 mm balloon was unsuccessful and the patient was referred for thoracoscopic myotomy. At the time of surgery, the left lower lobe of the lung was found to be fixed to the gastroesophageal junction by tumor that extended to involve the distal esophagus and pericardium. Biopsies showed infiltrating lobular breast carcinoma. The patient had a right mastectomy 15 years previously. The patient recovered from surgery and was treated with tamoxifen with gradual normalization of her swallowing function. She declined repeat manometric or radiological assessment.
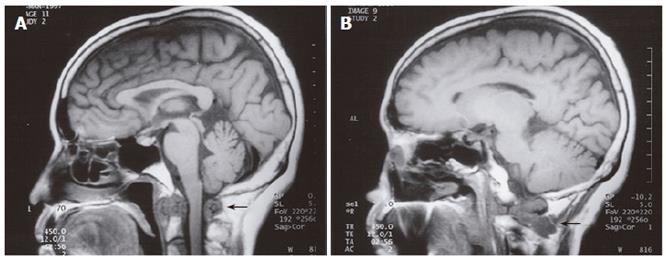
A 58-year old female with a previous history of Paget’s disease of the bone involving the cervical spine, multinodular goitre and neck trauma secondary to a motor vehicle accident presented with oropharyngeal dysphagia and mild dysarthria. Barium swallow demonstrated poor bolus formation, decreased laryngeal elevation, incomplete oropharyngeal clearance, and aspiration of barium. Electromyography was suspicious of a motor neuron disease, demonstrating paralysis of the left vocal cord, tongue fasciculations, and denervation of the left sternocleidomastoid. The patient underwent magnetic resonance imaging (MRI) which demonstrated a soft tissue density at the C2 level, as well as marrow replacement in several vertebrae (Figure 2A and 2B). Open biopsy of the soft tissue showed adenocarcinoma consistent with metastatic breast cancer. The patient had a modified left radical mastectomy for ductal adenocarcinoma followed by radiation therapy 20 years previously.
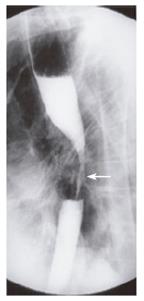
A 66-year old female presented with solid food dysphagia for several months. Barium swallow demonstrated a mid-esophageal stricture that was suspicious of neoplastic disease (Figure 3). Endoscopy and biopsy confirmed metastatic infiltration of the esophagus by lobular breast carcinoma. Unfortunately, attempted palliative dilatation resulted in perforation of the esophagus. The subsequent course of events resulted in prolonged hospitalization with multiple surgical procedures, complications and eventually death. The patient had a modified right mastectomy and radiotherapy 13 years previously for node negative malignancy. The patient had known metastatic disease to the left hilum, proven by biopsy, 6 years prior to the presentation of dysphagia.
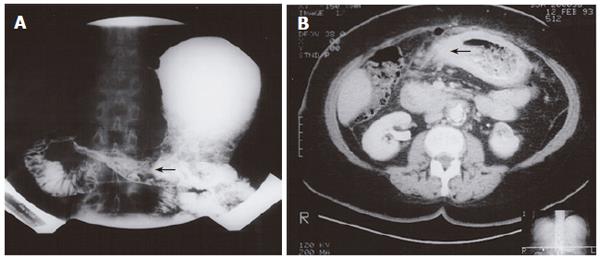
An 80-year old female presented with a history of early satiety and vomiting. Double contrast upper gastrointestinal barium examination was consistent with linitis plastica (Figure 4A). Endoscopy with biopsy of the gastric body and antrum confirmed metastatic infiltrating lobular carcinoma of breast origin. Subsequent computed tomography (CT) scanning demonstrated changes in the stomach and duodenum (Figure 4B). The patient received palliative care, but her symptoms were progressed after 14 mo. Plain films of the abdomen demonstrated a small bowel obstruction, suspected to be due to intra-abdominal spread of the disease. The patient underwent modified left radical mastectomy 2 years previously and had node positive disease.
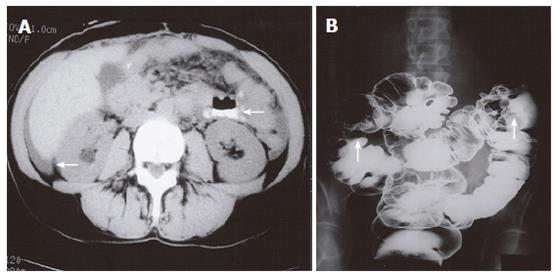
A 60-year old female presented with a history of diarrhea, abdominal cramps and vomiting. Crohn’s disease was suspected and she was initially treated with prednisone with clinical improvement. Several months later she experienced an exacerbation of her symptoms and presented with a partial small bowel obstruction. Small bowel enteroclysis demonstrated multiple abnormalities, including persistent narrowing of the gastric body and antrum (Figure 5), prominent irregular gastric rugae with diffuse mucosal serration, as well as 3 separate strictures of the ileum. The findings were compatible with the diagnosis of Crohn’s disease, but upper gastrointestinal endoscopy showed gross findings suspicious of malignancy. Biopsy demonstrated adenocarcinoma of breast origin. CT examination demonstrated ascites and mesenteric involvement, subsequently confirmed at laparotomy. The patient had a modified radical mastectomy 10 years previously.
A 51-year old female had a 3-year history of increasing abdominal pain. CT examination demonstrated a dilated small bowel as well as ascites (Figure 6A). Subsequent laparotomy demonstrated that the patient had a small bowel obstruction secondary to carcinomatosis consistent with metastatic breast cancer. After surgical treatment and hormonal therapy, the patient improved clinically. One year later, she presented with diarrhea. A double contrast barium enema demonstrated lesions in the ascending, distal transverse and sigmoid regions of the colon (Figure 6B). The appearance of the lesions was consistent with synchronous colonic cancers, but proved to be metastatic breast adenocarcinoma on pathology. The patient was diagnosed with breast cancer 4 years prior to the presentation with small bowel obstruction.
Breast cancer accounts for 19% of cancer deaths in women[1]. Current therapies have had a significant impact on the mortality and survival rates of breast cancer[3]. Prognosis is related to several factors including the presence of metastases with tumor histology being one of the predictors of metastatic spread. For example, tubular and mucinous carcinomas have a lower incidence of metastases and a better prognosis[4]. Lobular carcinoma, though less common and by mechanisms that are not clear, is more likely to metastasize to the gastrointestinal tract[4]. Although the gastrointestinal tract is a less common site for metastatic involvement by breast cancer, recognising the range of possible presentations is important for early and accurate diagnosis and treatment. The cases we described in this report highlight these features. Also, our patients presented with gastrointestinal manifestations after an average of 9.5 years and as long as 20 years of initial diagnosis. A 30-year interval between the original diagnosis and the presentation of metastatic disease has been reported in the literature[5]. Given the increased survival of breast cancer patients with current therapeutic regimes, more unusual presentations of metastatic disease, including involvement of the gastrointestinal tract can be anticipated[2,6,7], and hence this diagnosis should be considered in any woman with a previous history of the disease presenting with new gastrointestinal complaints.
Previous case reports and small series that have documented gastrointestinal involvement by metastatic breast cancer, together with the cases presented here, are summarized in Table 1. Any region of the gastrointestinal tract can be involved. However, oropharyngeal and esophageal involvement presenting as dysphagia is unusual[8]. Our series is unique in that it includes three examples of this: case 1 with pseudoachalasia, case 2 with oropharyngeal dysphagia secondary to nerve involvement, and case 3 with malignant esophageal stricture. In our review of the literature, this is only the third reported case of pseudoachalasia secondary to metastatic breast cancer[9,10]. As well, this is the first reported case of oropharyngeal dysphagia resembling case 2. There is a previous report of one case of metastases to the tongue[11]. Esophageal involvement most commonly presents as a short-segment of extrinsic obstruction in the mid-esophagus and has been reported to occur up to 22 years following the initial diagnosis of breast cancer[8]. Mediastinal and hilar lymphadenopathy may be present[12], and endoscopic biopsy may not provide the diagnosis if the obstruction is extrinsic and does not involve the mucosa. Long segment involvement of the esophagus, up to 20 cm, has been described[13]. Symptoms as well as radiographic findings may mimic radiation-induced or peptic stricture[12].
| Site | Presentation | Reference |
| Oropharynx | Oropharyngeal dysphagia | (Case 2) |
| Tongue lesion | 11 | |
| Esophagus | Stricture | (Case 3), 8, 13, 31-44 |
| Achalasia | (Case 1), 9, 10 | |
| Non-specific dysmotility | 30 | |
| Stomach | Linitis plastica | (Case 4), 5, 6, 45-52 |
| Obstruction/Stenosis | (Case 5), 19, 45 | |
| Polyp | 45, 53, 54 | |
| Ulcer/Erosion | 19, 45, 55, 56 | |
| Perforation | 57 | |
| Small intestine | Obstruction/Stenosis | (Case 4), (Case 6), 20, 58-61 |
| Multiple strictures/ Diffuse infilt | (Case 5) | |
| Colon | Obstruction/Stenosis | 25, 59, 62-65 |
| Asymptomatic abdominal Mass | 19 | |
| Multiple strictures/ Diffuse infilt | (Case 6), 66 | |
| Polyp | 67 | |
| Rectum | Obstruction/Stenosis | 15,25,26, 68 |
| Linitis plastica | 47 |
The stomach is the most common site of gastrointestinal involvement by metastatic breast cancer with an incidence of up to 15% at autopsy[12,14]. The usual presentation mimics linitis plastica and is typically due to lobular carcinoma[6], as illustrated by case 4. Findings include gastric wall thickening, rigidity and decreased peristalsis. Cases in which the linitis plastica-like presentation precedes the diagnosis of breast cancer have been documented[6]. In one series of 22 patients, endoscopy and biopsy could provide the correct diagnosis in only 13, most likely because tumor infiltration is deep to the mucosa[15]. Metastatic involvement of the stomach can mimic primary gastric neoplasms and inflammatory conditions such as peptic ulcer or gastritis[16]. Bleeding and perforation have also been reported[17-19].
Malignant neoplasms of the small intestine are uncommon and are usually primary adenocarcinomas[20]. Metastatic involvement of the small bowel can be due to malignant melanoma, or cancer of the colon, cervix, ovary or rarely esophagus[12,20]. Metastatic involvement of the small intestine is recognized at autopsy[21,22], but clinical presentation is uncommon. Patients can present with abdominal pain and diarrhea[20], similar to case 5, intussusception[12] or even appendicitis[7]. The terminal ileum is involved more often than the proximal small bowel, possibly due to the tracking of peritoneal fluid along the mesentery into the right lower quadrant. The clinical and radiological presentation can mimic Crohn’s disease, as illustrated by case 5. Metastatic disease to the duodenum has also been described[23].
Colonic involvement is common with some series showing colonic metastases in up to 12% of patients with breast cancer[22,24]. Carcinoma of the breast is the most frequent source of hematogenous metastases to the large bowel[2], but involvement via peritoneal and lymphatic spread also occurs[12]. As in the stomach, the lobular form of breast cancer is more likely to metastasise to the colon[2,4]. The presentation can be similar to primary neoplasia of the colon or can mimic Crohn’s disease, both clinically and radiologically[2,12,16,15]. Air contrast barium enema or CT may demonstrate diffuse or multifocal involvement[16]. Mucosal nodularity, stenosis, decreased distensibility, angulation and tethering are also characteristic findings[12]. Solitary apple-core lesions are less common[15]. Similar to the upper gastrointestinal tract, endoscopy and biopsy provide only a moderate diagnostic yield: 6 of 10 patients in one series[15]. Deep biopsy or laparotomy may be required for diagnosis[18,15]. Rectal involvement has also been described, including rectal stenosis secondary to metastatic breast cancer[25,26].
Other intra-abdominal sites of metastatic breast cancer that do not involve the gastrointestinal tract per se have been reported. Ascites, secondary to peritoneal carcinomatosis, is a common abnormality demonstrated by abdominal CT[27]. Metastatic spread to the hepatobiliary tract is well described[19,28]. Metastases to the diaphragm, the genitourinary tract, the retroperitoneum, the mesenteric lymph nodes, the abdominal wall and subcutaneous tissues have been reported[11,12,27]. Spread to the ovaries is identified histologically, but is not usually clinically evident or demonstrated with CT in the absence of other evidence for metastatic disease[29].
Metastatic breast cancer involving the gastrointestinal tract can produce a wide range of clinical and radiological presentations, often mimicking other gastrointestinal disorders. Given the high prevalence of this disease, breast cancer needs to be considered in any women presenting with new gastrointestinal complaints, especially those with a history of breast cancer, even if the initial diagnosis was made many years previously.
S- Editor Wang J L- Editor Wang XL E- Editor Bi L
| 1. | Rubin P. Clinical Oncology, 7th ed. WB Saunders Co. 1993;187-211. |
| 2. | Gifaldi AS, Petros JG, Wolfe GR. Metastatic breast carcinoma presenting as persistent diarrhea. J Surg Oncol. 1992;51:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Chu KC, Tarone RE, Kessler LG, Ries LA, Hankey BF, Miller BA, Edwards BK. Recent trends in U.S. breast cancer incidence, survival, and mortality rates. J Natl Cancer Inst. 1996;88:1571-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 271] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Borst MJ, Ingold JA. Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery. 1993;114:637-641; discussion 641-642. [PubMed] |
| 5. | Benfiguig A, Anciaux ML, Eugène CI, Benkémoun G, Etienne JC. [Gastric metastasis of breast cancer occurring after a cancer-free interval of 30 years]. Ann Gastroenterol Hepatol (Paris). 1992;28:175-177. [PubMed] |
| 6. | Cormier WJ, Gaffey TA, Welch JM, Welch JS, Edmonson JH. Linitis plastica caused by metastatic lobular carcinoma of the breast. Mayo Clin Proc. 1980;55:747-753. [PubMed] |
| 7. | Burney RE, Koss N, Goldenberg IS. Acute appendicitis secondary to metastatic carcinoma of the breast. A report and review of two cases. Arch Surg. 1974;108:872-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Varanasi RV, Saltzman JR, Krims P, Crimaldi A, Colby J. Breast carcinoma metastatic to the esophagus: clinicopathological and management features of four cases, and literature review. Am J Gastroenterol. 1995;90:1495-1499. [PubMed] |
| 9. | Herrera JL. Case report: esophageal metastasis from breast carcinoma presenting as achalasia. Am J Med Sci. 1992;303:321-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Rozman RW Jr, Achkar E. Features distinguishing secondary achalasia from primary achalasia. Am J Gastroenterol. 1990;85:1327-1330. [PubMed] |
| 11. | Fink I, Garb J. Carcinoma of the tip of the tongue: A case of metastasis from a malignant tumor of the breast. Am J Surg. 1943;62:138-141. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Chang SF, Burrell MI, Brand MH, Garsten JJ. The protean gastrointestinal manifestations of metastatic breast carcinoma. Radiology. 1978;126:611-617. [PubMed] |
| 13. | Vansant JR, Davis RK. Esophageal obstruction secondary to mediastinal metastasis from breast carcinoma. Chest. 1971;60:93-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Joffe N. Metastatic involvement of the stomach secondary to breast carcinoma. Am J Roentgenol Radium Ther Nucl Med. 1975;123:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Taal BG, den Hartog Jager FC, Steinmetz R, Peterse H. The spectrum of gastrointestinal metastases of breast carcinoma: II. The colon and rectum. Gastrointest Endosc. 1992;38:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Easter DW, Jamshidipour R, McQuaid K. Laparoscopy to correctly diagnose and stage metastatic breast cancer mimicking Crohn's disease. Surg Endosc. 1995;9:820-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Davis HL Jr, Murray RK, Korbitz BC. Breast carcinoma metastatic to the stomach. Report of a case in a male and review of an autopsy series. Am J Dig Dis. 1968;13:868-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Choi SH, Sheehan FR, Pickren JW. Metastic involvement of the stomach by breast cancer. Cancer. 1964;17:791-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Schwarz RE, Klimstra DS, Turnbull AD. Metastatic breast cancer masquerading as gastrointestinal primary. Am J Gastroenterol. 1998;93:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 119] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Hansen RM, Lewis JD, Janjan NA, Komorowski RA. Occult carcinoma of the breast masquerading as primary adenocarcinoma of the small intestine. A case report. J Clin Gastroenterol. 1988;10:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Asch MJ, Wiedel PD, Habif DV. Gastrointestinal metastases from crcinoma of the breast. Autopsy study and 18 cases requiring operative intervention. Arch Surg. 1968;96:840-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 69] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Cifuentes N, Pickren JW. Metastases from carcinoma of mammary gland: an autopsy study. J Surg Oncol. 1979;11:193-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 124] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Hartmann WH, Sherlock P. Gastroduodenal metastases from carcinoma of the breast. An adrenal steroid-induced phenomenon. Cancer. 1961;14:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Graham WP, Goldman L. Gastrointestinal Metastases from Carcinoma of the Breast. Ann Surg. 1964;159:477-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Bamias A, Baltayiannis G, Kamina S, Fatouros M, Lymperopoulos E, Agnanti N, Tsianos E, Pavlidis N. Rectal metastases from lobular carcinoma of the breast: report of a case and literature review. Ann Oncol. 2001;12:715-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Cervi G, Vettoretto N, Vinco A, Cervi E, Villanacci V, Grigolato P, Giulini SM. Rectal localization of metastatic lobular breast cancer: report of a case. Dis Colon Rectum. 2001;44:453-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Caskey CI, Scatarige JC, Fishman EK. Distribution of metastases in breast carcinoma: CT evaluation of the abdomen. Clin Imaging. 1991;15:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Franco D, Martin B, Smadja C, Szekely AM, Rougier P. Biliary metastases of breast carcinoma. The case for resection. Cancer. 1987;60:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Meyer JI, Herts BR, Einstein DM, Singer AA, Budd GT, Cohen MA. Pelvic computed tomography of breast carcinoma patients. Should it routinely be added to abdominal computed tomography. Cancer. 1997;79:500-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Isaacs P, MacGillivray N, Springett P. Late recurrence of breast cancer presenting with esophageal dysmotility. J Clin Gastroenterol. 1989;11:588-590. [PubMed] [DOI] [Full Text] |
| 31. | Goldberg RI, Rams H, Stone B, Barkin JS. Dysphagia as the presenting symptom of recurrent breast carcinoma. Cancer. 1987;60:1085-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Biller HF, Diktaban T, Fink W, Lawson W. Breast carcinoma metastasizing to the cervical esophagus. Laryngoscope. 1982;92:999-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Orringer MB, Skinner DB. Unusual presentations of primary and secondary esophageal malignancies. Ann Thorac Surg. 1971;11:305-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Conklin EF. Some unusual complications of metastatic carcinoma of the breast. Ann Surg. 1964;139:489-495. |
| 35. | Stallone RJ, Roe BB. Breast carcinoma as a cause of dysphagia. Dis Chest. 1969;56:449-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Polk HC Jr, Camp FA, Walker AW. Dysphagia and esophageal stenosis: manifestation of metastatic mammary cancer. Cancer. 1967;20:2002-2007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Atkins JP. Metastatic carcinoma to the esophagus. Endoscopic considerations with special reference to carcinoma of the breast. Ann Otol Rhinol Laryngol. 1966;75:356-367. [PubMed] |
| 38. | Phadke M, Rao U, Takita H. Metastatic tumors of esophagus. N Y State J Med. 1976;76:963-965. [PubMed] |
| 39. | Fujii K, Nakanishi Y, Ochiai A, Tsuda H, Yamaguchi H, Tachimori Y, Kato H, Watanabe H, Shimoda T. Solitary esophageal metastasis of breast cancer with 15 years' latency: a case report and review of the literature. Pathol Int. 1997;47:614-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Wu CM, Hruban RH, Fishman EK. Breast carcinoma metastatic to the esophagus. CT findings with pathologic correlation. Clin Imaging. 1998;22:343-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Toreson WE. Secondary carcinoma of the esophagus as a cause of dysphagia. Arch Pathol. 1944;38:82–84. |
| 42. | Shimada Y, Imamura M, Tobe T. Successful esophagectomy for metastatic carcinoma of the esophagus from breast cancer--a case report. Jpn J Surg. 1989;19:82-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Simchuk EJ, Low DE. Direct esophageal metastasis from a distant primary tumor is a submucosal process: a review of six cases. Dis Esophagus. 2001;14:247-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Vergote G, Ponette E, Verschakelen J, Baert AL, Rutgeerts P, Moerman P. Esophageal metastasis from breast carcinoma. J Belge Radiol. 1994;77:268-269. [PubMed] |
| 45. | Taal BG, Peterse H, Boot H. Clinical presentation, endoscopic features, and treatment of gastric metastases from breast carcinoma. Cancer. 2000;89:2214-2221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 46. | Ferri LE, Onerheim R, Emond C. Linitis plastica as the first indication of metastatic lobular carcinoma of the breast: case report and literature review. Can J Surg. 1999;42:466-469. [PubMed] |
| 47. | Clavien PA, Laffer U, Torhost J, Harder F. Gastro-intestinal metastases as first clinical manifestation of the dissemination of a breast cancer. Eur J Surg Oncol. 1990;16:121-126. [PubMed] |
| 48. | Lorimier G, Binelli C, Burtin P, Maillart P, Bertrand G, Verriele V, Fondrinier E. Metastatic gastric cancer arising from breast carcinoma: endoscopic ultrasonographic aspects. Endoscopy. 1998;30:800-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Shimizu S, Matsukawa H, Iwasaki H, Fukagawa H, Masukawa K, Horiguchi K, Ohaki Y. [A case of gastric metastases from carcinoma of the breast]. Gan No Rinsho. 1988;34:1163-1168. [PubMed] |
| 50. | Flentje M, Rieden K. [Linitis plastica of the stomach in breast cancer]. Rontgenblatter. 1988;41:66-67. [PubMed] |
| 51. | Rodde A, Stines J, Regent D, Becker S, Conroy T, Weber B, Delgoffe C, Bour C. [Pseudolinitis plastica of breast origin]. J Radiol. 1987;68:269-274. [PubMed] |
| 52. | Elliott LA, Hall GD, Perren TJ, Spencer JA. Metastatic breast carcinoma involving the gastric antrum and duodenum: computed tomography appearances. Br J Radiol. 1995;68:970-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 53. | Karamlou TB, Vetto JT, Corless C, Deloughery T, Faigel D, Blanke C. Metastatic breast cancer manifested as refractory anemia and gastric polyps. South Med J. 2002;95:922-925. [PubMed] |
| 54. | Shimizu M, Matsumoto T, Hirokawa M, Shimozuma K, Manabe T. Gastric metastasis from breast cancer: a pitfall in gastric biopsy specimens. Pathol Int. 1998;48:240-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 55. | Reiman T, Butts CA. Upper gastrointestinal bleeding as a metastatic manifestation of breast cancer: a case report and review of the literature. Can J Gastroenterol. 2001;15:67-71. [PubMed] |
| 56. | Pera M, Riera E, Lopez R, Viñolas N, Romagosa C, Miquel R. Metastatic carcinoma of the breast resembling early gastric carcinoma. Mayo Clin Proc. 2001;76:205-207. [PubMed] |
| 57. | Ghosn M, Ghayad E, Biagini J, Abi Gerges D. [Hypothalamo-hypophyseal and gastric metastasis of a breast neoplasm. Clinical case and a review of the literature]. Bull Cancer. 1991;78:1071-1073. [PubMed] |
| 58. | Lottini M, Neri A, Vuolo G, Testa M, Pergola L, Cintorino M, Guarnieri A. Duodenal obstruction from isolated breast cancer metastasis: a case report. Tumori. 2002;88:427-429. [PubMed] |
| 59. | Daniels IR, Layer GT, Chisholm EM. Bowel obstruction due to extrinsic compression by metastatic lobular carcinoma of the breast. J R Soc Promot Health. 2002;122:61-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 60. | Houghton AD, Pheils P. Isolated duodenal metastasis from breast carcinoma. Eur J Surg Oncol. 1987;13:367-369. [PubMed] |
| 61. | Sarkar N, Kejariwal D, Roy S. Isolated duodenal metastasis from breast carcinoma. J Assoc Physicians India. 2002;50:962-963. [PubMed] |
| 62. | Yokota T, Kunii Y, Kagami M, Yamada Y, Takahashi M, Kikuchi S, Nakamura M, Yamauchi H. Metastatic breast carcinoma masquerading as primary colon cancer. Am J Gastroenterol. 2000;95:3014-3016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Koutsomanis D, Renier JF, Ollivier R, Moran A, el-Haite AA. Colonic metastasis of breast carcinoma. Hepatogastroenterology. 2000;47:681-682. [PubMed] |
| 64. | Eyres KS, Sainsbury JR. Large bowel obstruction due to metastatic breast cancer: an unusual presentation of recurrent disease. Br J Clin Pract. 1990;44:333-334. [PubMed] |
| 65. | Koop H, Dombrowski H, Maroske D, Schwerk WB, Schmitz-Moormann P, Arnold R. [Segmental colonic stenosis in intestinal metastasis of breast carcinoma. A contribution to the differential diagnosis of colitis]. Dtsch Med Wochenschr. 1988;113:1101-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 66. | Rabau MY, Alon RJ, Werbin N, Yossipov Y. Colonic metastases from lobular carcinoma of the breast. Report of a case. Dis Colon Rectum. 1988;31:401-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 67. | Vaidya JS, Mukhtar H, Bryan R. Colonic metastasis from a breast cancer--a case report and a few questions. Eur J Surg Oncol. 2002;28:463-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 68. | Nieboer P, van der Graaf WT, de Knegt RJ, van Dullemen HM. Rectal syndrome as first presentation of metastatic breast cancer. Am J Gastroenterol. 2000;95:2138-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |