Published online Oct 14, 2006. doi: 10.3748/wjg.v12.i38.6182
Revised: April 28, 2006
Accepted: May 25, 2006
Published online: October 14, 2006
AIM: To investigate the role of vascular endothelial growth factor (VEGF) and its receptors VEGFR-1 and 2 in the growth and differentiation of gastrointestinal stromal tumors (GISTs).
METHODS: Thirty-three GISTs, 15 leiomyomas and 6 schwannomas were examined by immunohistochemistry in this study.
RESULTS: VEGF protein was expressed in the cytoplasm of tumor cells, and VEGFR-1 and 2 were expressed both in the cytoplasm and on the membrane of all tumors. Immunohistochemical staining revealed that 26 GISTs (78.8%), 9 leiomyomas (60.0%) and 3 schwannomas (50.0%) were positive for VEGF; 24 GISTs (72.7%), 12 leiomyomas (80.0%) and 4 schwannomas (66.7%) were positive for VEGFR-1; 30 GISTs (90.9%), 5 leiomyomas (33.3%) and 4 schwannomas (66.7%) were positive for VEGFR-2. VEGFR-2 expression was statistically different between GISTs and leiomyomas (P < 0.0001). However, there was no correlation between the expression of VEGF pathway componenets and the clinical risk categories.
CONCLUSION: Our results suggest that the VEGF pathway may play an important role in the differentiation of GISTs, leiomyomas and schwannomas.
- Citation: Nakayama T, Cho YC, Mine Y, Yoshizaki A, Naito S, Wen CY, Sekine I. Expression of vascular endothelial growth factor and its receptors VEGFR-1 and 2 in gastrointestinal stromal tumors, leiomyomas and schwannomas. World J Gastroenterol 2006; 12(38): 6182-6187
- URL: https://www.wjgnet.com/1007-9327/full/v12/i38/6182.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i38.6182
Gastrointestinal stromal tumors (GISTs) are rare mesenchymal tumors of the gastrointestinal (GI) tract that may occur from the oesophagus to the anus, including the omentum[1,2]. Despite their rarity, GISTs are the most common primary mesenchymal tumors of the GI tract[1-3]. The mechanisms of tumourigenesis, progression and differentiation of GISTs are unknown. Traditionally, all primary mesenchymal spindle cell tumors of the GI tract are uniformly classified as smooth muscle tumors (e.g., leiomyomas, cellular leiomyomas or leiomyosarcomas). Tumors with epithelioid cytologic features are designated leiomyoblastomas or epithelioid leiomyosarcomas[4]. Recently, Sircar et al[5] postulated that GISTs originate from Cajal cells in the GI tract and differ from leiomyomas and schwannomas, which are of mesenchymal cell origin. Cajal cells are thought to be gastrointestinal pacemaker cells that regulate intestinal motility[6]. GISTs are characterized by frequent expression of the bone marrow leukocytic progenitor cell antigen CD34[7] and the c-kit proto-oncogene[2,3,5].
Some GISTs have mutations in the genes encoding c-kit and platelet-derived growth factor alpha (PDGFR-α) that cause constitutive tyrosine kinase activation[3,8-10]. Tumors expressing c-kit or PDGFR-α oncoproteins are indistinguishable with respect to activation of downstream signaling intermediates and cytogenetic changes associated with tumor progression. C-kit and PDGFR-α mutations appear to be alternative and mutually exclusive oncogenic mechanisms in GISTs[9,10].
Vascular endothelial growth factor (VEGF) has been identified as a key regulator of tumor angiogenesis, and VEGF receptors (VEGFR) are the major mediators of the mitogenic and permeability-enhancing effects of VEGF in endothelial cells[11,12]. In addition, VEGF is a survival factor for endothelial cells, and a marked dependence on VEGF has been shown in newly formed but not established tumor vessels[13]. Although the field of tumor angiogenesis is an area of extensive research, the consequences of enhanced angiogenesis and its reversion on tumor growth and progression are only partially elucidated[14-16]. Recently, coexpression of VEGF and its receptor, either VEGFR-1 (Flt-1) or VEGFR-2 (Flk-1/KDR), has been reported in tumor cells, suggesting the presence of an autocrine and/or a paracrine VEGF/VEGFR growth pathway in solid tumors[17-19]. Further, the expression levels of VEGF and its receptors have been shown to correlate with progressive tumor growth and development of metastasis by many carcinomas[20].
These studies suggest that the VEGF pathway is involved in tumor growth and differentiation. However, there are no data detailing VEGFR expression in GISTs, leiomyomas or schwannomas, or the role of VEGF in the etiology of these tumors. The purpose of this study was to investigate the expression of VEGF and VEGFRs in GISTs.
Thirty-three specimens of GISTs (28 from the stomach and 5 from the small intestine), 15 specimens of leiomyomas (4 from the oesophagus, 4 from the stomach and 7 from the large intestine), 6 specimens of schwannomas (5 from the stomach and 1 from the large intestine) were selected from surgical pathology archival tissues at Nagasaki University Hospital between 2001 and 2006. The specimens of GISTs, leiomyomas and schwannomas were 0.8-12.0 cm, 0.1-4.5 cm and 0.6-5.0 cm, respectively. In this study, GISTs were defined as expressing both c-kit and CD34 surface antigens and classified by risk categories, mitosis counts and tumor size[21]. The number of mitoses was determined by counting 50 high-power fields (HPF, × 400) under a Nikon (Tokyo, Japan) E400 microscope. Leiomyomas were defined both as expressing α-smooth muscle cell actin (SMA) but not c-kit, CD34 or S100-protein and as expressing S100-protein but not c-kit, CD34 or SMA. Two independent pathologists (T. Nakayama and I. Sekine) determined tumor identification/classification.
The subcellular localization of VEGF, VEGFR-1 and 2 was determined in GISTs using polyclonal antibodies directed against unique sequences of VEGF, VEGFR-1 and 2. These antibodies were devoid of any cross-reaction with other proteins in the VEGF family. Formalin-fixed and paraffin-embedded specimens were cut into 4 μm thick sections, deparaffinized and preincubated with normal bovine serum to prevent non-specific binding. The sections were incubated overnight at 4°C with primary polyclonal antibody to human VEGF [(147), 1 mg/L; Santa Cruz Biotechnology Inc., Santa Cruz, CA], VEGFR-1 [Flt-1(C-17), 1 mg/L; Santa Cruz Biotechnology Inc.] or VEGFR-2 [Flk-1(C-20), 1 mg/L; Santa Cruz Biotechnology Inc.], followed by alkaline phosphatase-conjugated goat anti-rabbit IgG antibody (0.4 μg/mL; Santa Cruz Biotechnology, Inc.). The reaction products were visualized using a mixture of 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium chloride (BCIP/NBT; Roche Diagnostic Corp., Indianapolis, IN). Negative controls replaced the primary antibody with non-immunized rabbit serum, and human breast cancer tissue served as the positive control[22]. VEGF, VEGFR-1 and 2 expressions were classified into 3 categories depending upon the percentage of cells stained and/or the intensity of staining ( -: 0% to 15% positive tumor cells; +: > 15% positive tumor cells).
The Stat View II program (Abacus Concepts, Inc., Berkeley, CA) was used for statistical analysis. Analyses comparing the degree of VEGF, VEGFR-1 or 2 expressions in GISTs, leiomyomas and schwannomas were performed using the Mann-Whitney’s test.
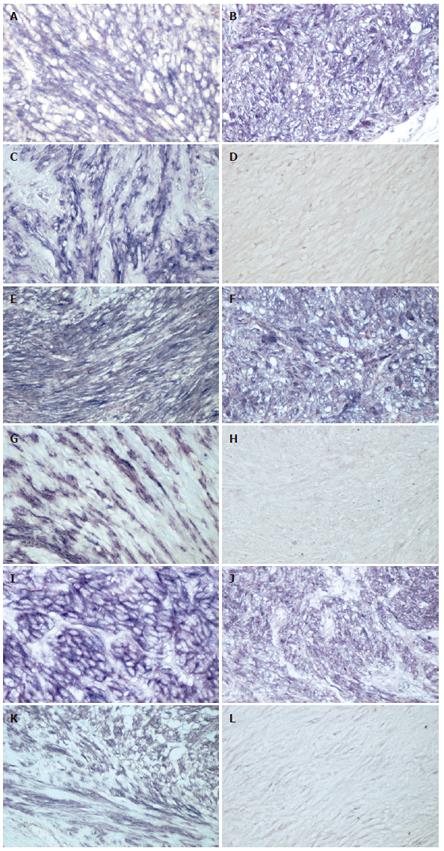
The results of immunohistochemical stainings for VEGF, VEGFR-1 or 2 are summarized in Table 1. VEGF, VEGFR-1 and 2 expression was heterogeneous in GISTs, leiomyomas and schwannomas and localized to the cytoplasm and/or membrane of tumor cells (Figure 1). Immunohistochemical staining revealed VEGF expression in the cytoplasm of GIST (Figure 1A), leiomyoma (Figure 1B) and schwannoma (Figure 1C) cells. VEGFR-1 expression was shown in the membrane and cytoplasm of GIST (Figure 1E), leiomyoma (Figure 1F) and schwannoma (Figure 1G) cells. VEGFR-2 was expressed in the membrane and cytoplasm of GIST (Figure 1I), leiomyoma (Figure 1J) and schwannoma (Figure 1K) cells. Immunohistochemical staining was positive for VEGF in 26 (78.8%) of 33 GISTs, 9 (60.0%) of 15 leiomyomas and 3 (50.0%) of 6 schwannomas, respectively. Twenty-four (72.7%) of GISTs, 12 (80.0%) of leiomyomas and 4 (66.7%) of schwannomas showed positive staining for VEGFR-1. There was no statistical difference in VEGF or VEGFR-1 expression between GISTs and leiomyomas or schwannomas. Immunohistochemical staining was positive for VEGFR-2 in 30 (90.9%) of GISTs, 5 (33.3%) of 15 leiomyomas an 4 (66.7%) of schwannomas. There was a statistical difference in VEGFR-2 expression between GISTs and leiomyomas (P < 0.0001).
| n | VEGF | VEGFR-1 | VEGFR-2 | ||||
| - | + | - | + | - | + | ||
| GISTs | 33 | 7 (21.2) | 26 (78.8) | 9 (27.3) | 24 (72.7) | 3 (9.1) | 30 (90.9) b |
| Leiomyoma | 15 | 6 (40.0) | 9 (60.0) | 3 (20.0) | 12 (80.0) | 10 (66.7) | 5 (33.3) |
| Schwannoma | 6 | 3 (50.0) | 3 (50.0) | 2 (33.3) | 4 (66.7) | 2 (33.3) | 4 (66.7) |
The classification of GISTs by risk category, mitosis counts and tumor size is shown in Table 2. All 4 cases within the high risk category expressed VEGF, VEGFR-1 and 2. All 4 cases with over 10 mitoses per 50 HPFs strongly expressed VEGF, VEGFR-1 and 2. Finally, only one tumor that measured over 10 cm strongly expressed VEGF, VEGFR-1 and 2. However, there was no correlation between VEGF, VEGFR-1 or VEGFR-2 expression and each classification.
| Total | n | VEGF | VEGFR-1 | VEGFR-2 | |||
| - | + | - | + | - | + | ||
| 33 | 7 | 26 | 9 | 24 | 3 | 30 | |
| Risk categories | NS | NS | NS | ||||
| High | 4 | 0 (0.0) | 4 (100) | 0 (0.0) | 4 (100) | 0 (0.0) | 4 (100) |
| Intermediate | 5 | 1 (20.0) | 4 (80.0) | 2 (40.0) | 3 (60.0) | 0 (0.0) | 5 (100) |
| Low | 17 | 3 (17.6) | 14 (82.4) | 5 (29.4) | 12 (70.6) | 2 (11.8) | 15 (88.2) |
| Very low | 7 | 3 (42.9) | 4 (57.1) | 2 (28.6) | 5 (71.4) | 1 (14.3) | 6 (85.7) |
| Mitosis counts (per 50 fields, HPF) | NS | NS | NS | ||||
| < 2 | 17 | 4 (23.5) | 13 (76.5) | 6 (35.3) | 11 (64.7) | 2 (11.8) | 15 (88.2) |
| 2-5 | 8 | 2 (25.0) | 6 (75.0) | 3 (37.5) | 5 (62.5) | 1 (12.5) | 7 (87.5) |
| 6-10 | 4 | 1 (25.0) | 3 (75.0) | 0 (0.0) | 4 (100) | 0 (0.0) | 4 (100) |
| >10 | 4 | 0 (0.0) | 4 (100) | 0 (0.0) | 4 (100) | 0 (0.0) | 4 (100) |
| Tumour size (cm in length) | NS | NS | NS | ||||
| < 2 | 6 | 3 (50.0) | 3 (50.0) | 2 (33.3) | 4 (66.7) | 1 (16.7) | 5 (83.3) |
| 2-< 5 | 20 | 3 (15.0) | 17 (85.0) | 5 (25.0) | 15 (75.0) | 2 (10.0) | 18 (90.0) |
| 5-< 10 | 6 | 1 (16.7) | 5 (83.3) | 2 (33.3) | 4 (66.7) | 0 (0.0) | 6 (100) |
| > 10 | 1 | 0 (0.0) | 1 (100) | 0 (0.0) | 1 (100) | 0 (0.0) | 1 (100) |
The coexpression of VEGF and VEGFR-1 and 2 has been reported in tumor cells, suggesting the presence of an autocrine and/or a paracrine VEGF/VEGFR growth pathway in solid tumors[17-19]. VEGF also has been shown to play a role in the proliferation and/or differentiation of stromal tumors and normal mesenchymal cells[23-26]. VEGF expression in GISTs has been already reported[27,28]. However, there are no studies on VEGF receptor expression in GISTs, leiomyomas and schwannomas, or on the potential roles of VEGF and its receptors in the growth of these tumors. This is the first study to determine the expression of VEGF receptors in GIST and stromal tumors, demonstrating substantial levels of VEGF and its receptors in the cytoplasm of GIST, leiomyoma and schwannoma cells. Therefore, we suggest that VEGF and its receptors may play an important role in the growth and/or differentiation of intestinal stromal tumors via autocrine and/or paracrine pathways.
We did not find any statistical correlation between risk grade and the expression of VEGF or VEGFRs for GISTs. However, all 4 GISTs in the high risk category expressed VEGF and VEGFRs (Table 2). Furthermore, all 4 GISTs that had higher mitosis counts (over ten per 50 HPFs) were positive for VEGF and VEGFRs. Our data suggest that high risk GIST- expressed VEGF and VEGFR level is higher than normal. We thought that the number of high risk GISTs should be less. Further studies are needed to examine the VEGF/VEGFR pathway components in high risk GISTs.
VEGF induces a variety of enzymes and proteins important in the degradation process, including matrix-degrading metalloproteinase, interstitial collagenase, and serine proteases such as urokinase-type plasminogen activator (u-PA) and tissue-type plasminogen activator (TTPA)[29,30]. In this study, we did not evaluate the invasive activities of GIST cells, because all the GISTs were solitary and showed clear margins. However, the activation of these factors by VEGFRs provides for the possibility of conduction to a prodegradative environment that facilitates migration and invasion of tumor cells.
Solid tumors develop regions of low oxygen tension because of an imbalance in oxygen supply and consumption. Hypoxia in the tumor microenvironment is sufficient to activate hypoxia-inducible factor (HIF)-dependent gene expression[31]. HIF-1 alpha (HIF-1α) is overexpressed in most human malignancies[32]. HIF-1 binds to HIF responsive elements in the promoter region of certain genes, such as VEGF, to increase transcription[33]. HIF-1 expression in GIST has been already reported and suggested to contribute to tumor angiogenesis in GIST[28]. HIF-1 might play a role in the growth of GIST, because VEGF was expressed greater in larger GISTs in this study. However, we do not have any data about hypoxia, angiogenesis or HIF-1 expression in GIST, because the purpose of this study was to clarify the role of VEGF/VEGFR pathway in GIST cells. We hope that the relationship between hypoxia and GIST growth can be clarified in next study.
Joensuu et al[34] have reported a patient in whom Imatinib (STI-571, Gleevec), a tyrosine kinase inhibitor, is effective against GIST. Imatinib has proven to be remarkably efficacious in heavily pretreated GIST patients with advanced disease in phase III clinical trials[35]. It was reported that Imatinib down-regulates VEGF expression in the GIST cell line GIST-T1[24]. Furthermore, anti-VEGF reagents are used in clinical trials for the therapy of colorectal, lung and breast cancer[36]. Stimulation of VEGFR upregulates the mitogen-activated protein kinase pathway through the activation of tyrosine kinases[37,38], the same pathway utilized by c-kit activation. These anti-VEGF reagents might be useful for the therapy of GISTs via the down-regulation of the VEGF/VEGFR pathway.
We are grateful to Mr. Toshiyuki Kawada (Nagasaki University Graduate School of Biomedical Sciences) for his excellent immunohistochemical assistance.
S- Editor Wang J L- Editor Wang XL E- Editor Liu WF
| 1. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1178] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 2. | Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259-1269. [PubMed] |
| 3. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3114] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 4. | Appelman HD. Mesenchymal tumors of the gut: historical perspectives, new approaches, new results, and does it make any difference. Monogr Pathol. 1990;220-246. [PubMed] |
| 5. | Sircar K, Hewlett BR, Huizinga JD, Chorneyko K, Berezin I, Riddell RH. Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol. 1999;23:377-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 337] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 6. | Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 765] [Cited by in RCA: 747] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 7. | Miettinen M, Virolainen M. Gastrointestinal stromal tumors--value of CD34 antigen in their identification and separation from true leiomyomas and schwannomas. Am J Surg Pathol. 1995;19:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 292] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 8. | Plaat BE, Hollema H, Molenaar WM, Torn Broers GH, Pijpe J, Mastik MF, Hoekstra HJ, van den Berg E, Scheper RJ, van der Graaf WT. Soft tissue leiomyosarcomas and malignant gastrointestinal stromal tumors: differences in clinical outcome and expression of multidrug resistance proteins. J Clin Oncol. 2000;18:3211-3220. [PubMed] |
| 9. | Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1712] [Cited by in RCA: 1722] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 10. | Hirota S, Ohashi A, Nishida T, Isozaki K, Kinoshita K, Shinomura Y, Kitamura Y. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology. 2003;125:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 478] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 11. | Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2935] [Cited by in RCA: 2882] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 12. | Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1091] [Cited by in RCA: 1110] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 13. | Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 859] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 14. | Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6437] [Cited by in RCA: 6489] [Article Influence: 259.6] [Reference Citation Analysis (0)] |
| 15. | Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999;5:1359-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 726] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 16. | Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029-1039. [PubMed] |
| 17. | Kim SJ, Seo JH, Lee YJ, Yoon JH, Choi CW, Kim BS, Shin SW, Kim YH, Kim JS. Autocrine vascular endothelial growth factor/vascular endothelial growth factor receptor-2 growth pathway represents a cyclooxygenase-2-independent target for the cyclooxygenase-2 inhibitor NS-398 in colon cancer cells. Oncology. 2005;68:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Yokoi K, Thaker PH, Yazici S, Rebhun RR, Nam DH, He J, Kim SJ, Abbruzzese JL, Hamilton SR, Fidler IJ. Dual inhibition of epidermal growth factor receptor and vascular endothelial growth factor receptor phosphorylation by AEE788 reduces growth and metastasis of human colon carcinoma in an orthotopic nude mouse model. Cancer Res. 2005;65:3716-3725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Guo P, Fang Q, Tao HQ, Schafer CA, Fenton BM, Ding I, Hu B, Cheng SY. Overexpression of vascular endothelial growth factor by MCF-7 breast cancer cells promotes estrogen-independent tumor growth in vivo. Cancer Res. 2003;63:4684-4691. [PubMed] |
| 20. | Barozzi C, Ravaioli M, D'Errico A, Grazi GL, Poggioli G, Cavrini G, Mazziotti A, Grigioni WF. Relevance of biologic markers in colorectal carcinoma: a comparative study of a broad panel. Cancer. 2002;94:647-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 275] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 22. | Garvin S, Nilsson UW, Dabrosin C. Effects of oestradiol and tamoxifen on VEGF, soluble VEGFR-1, and VEGFR-2 in breast cancer and endothelial cells. Br J Cancer. 2005;93:1005-1010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Frick M, Dulak J, Cisowski J, Józkowicz A, Zwick R, Alber H, Dichtl W, Schwarzacher SP, Pachinger O, Weidinger F. Statins differentially regulate vascular endothelial growth factor synthesis in endothelial and vascular smooth muscle cells. Atherosclerosis. 2003;170:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Jin T, Nakatani H, Taguchi T, Nakano T, Okabayashi T, Sugimoto T, Kobayashi M, Araki K. STI571 (Glivec) suppresses the expression of vascular endothelial growth factor in the gastrointestinal stromal tumor cell line, GIST-T1. World J Gastroenterol. 2006;12:703-708. [PubMed] |
| 25. | Tomanek RJ, Ishii Y, Holifield JS, Sjogren CL, Hansen HK, Mikawa T. VEGF family members regulate myocardial tubulogenesis and coronary artery formation in the embryo. Circ Res. 2006;98:947-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Kria L, Khalfaoui T, Mkannez G, Beltaief O, Anane R, Errais K, Tounsi L, Zhioua R, Jilani SB, Ouertani AM. Immunohistochemical study of vascular endothelial growth factor (VEGF), tumor suppressor protein (p53) and intercellular adhesion molecule (ICAM-1) in the conjunctiva of diabetic patients. J Mol Histol. 2005;36:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Takahashi R, Tanaka S, Kitadai Y, Sumii M, Yoshihara M, Haruma K, Chayama K. Expression of vascular endothelial growth factor and angiogenesis in gastrointestinal stromal tumor of the stomach. Oncology. 2003;64:266-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Chen WT, Huang CJ, Wu MT, Yang SF, Su YC, Chai CY. Hypoxia-inducible factor-1alpha is associated with risk of aggressive behavior and tumor angiogenesis in gastrointestinal stromal tumor. Jpn J Clin Oncol. 2005;35:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res. 2001;49:568-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 484] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 30. | Choong PF, Nadesapillai AP. Urokinase plasminogen activator system: a multifunctional role in tumor progression and metastasis. Clin Orthop Relat Res. 2003;S46-S58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 131] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Nakashima T, Kondoh S, Kitoh H, Ozawa H, Okita S, Harada T, Shiraishi K, Ryozawa S, Okita K. Vascular endothelial growth factor-C expression in human gallbladder cancer and its relationship to lymph node metastasis. Int J Mol Med. 2003;11:33-39. [PubMed] |
| 32. | Yue SQ, Yang YL, Zhou JS, Li KZ, Dou KF. Relationship between urokinase-type plasminogen activator receptor and vascular endothelial growth factor expression and metastasis of gallbladder cancer. World J Gastroenterol. 2004;10:2750-2752. [PubMed] |
| 33. | Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604-4613. [PubMed] |
| 34. | Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1430] [Cited by in RCA: 1326] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 35. | Maki RG. Gastrointestinal Stromal Tumors Respond to Tyrosine Kinase-targeted Therapy. Curr Treat Options Gastroenterol. 2004;7:13-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 792] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 37. | de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1469] [Cited by in RCA: 1459] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 38. | Quinn TP, Peters KG, De Vries C, Ferrara N, Williams LT. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci USA. 1993;90:7533-7537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 559] [Article Influence: 17.5] [Reference Citation Analysis (0)] |