Published online Oct 7, 2006. doi: 10.3748/wjg.v12.i37.6059
Revised: May 28, 2006
Accepted: June 16, 2006
Published online: October 7, 2006
This case report describes a young female patient presenting with acute intra-abdominal hemorrhage originating from a large tumor in the liver, most likely a hepatocellular adenoma. The bleeding was stopped by selective embolization of right hepatic artery branches. Subsequently, partial hepatectomy was performed after 6 mo. Macro- and microscopic examination showed complete necrosis and absence of tumorous tissue. The patient was discharged without complications, and subsequent follow-up until 22 mo after resection did not reveal any new lesions in the liver. This case emphasizes the significance of selective arterial embolization in the management of bleeding liver tumors and questions the need for (partial) hepatectomy after this procedure in selective cases.
- Citation: Huurman VA, Stoot JH, Linden EVD, Terpstra OT, Schaapherder AF. Necrosis of a large hepatic tumor after hemorrhage and subsequent selective arterial embolization. World J Gastroenterol 2006; 12(37): 6059-6061
- URL: https://www.wjgnet.com/1007-9327/full/v12/i37/6059.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i37.6059
Hepatocellular adenomas are uncommon benign liver tumours that are present mainly in women of reproductive age[1]. Their existence is associated with the use of oral contraceptives[2]. The first clinical presentation of the tumour can consist of abdominal pain, hepatomegaly, or hepatic (subcapsular) hemorrhage and shock[1]. For the latter presentation, emergency surgery by partial hepatectomy is the current treatment of choice. However, this treatment is associated with high morbidity and mortality rates[3,4]. Therefore, several groups suggest a role for initial conservative treatment[1,5].
Selective arterial embolization is a novel method for the management of intra-abdominal hemorrhage without the need for major surgery, and our hospital has gained considerable experience with this procedure. However, the use of this elegant method when coping with liver hemorrhage has only been described incidentally[1,5,6].
After any initial emergency treatment, secondary partial hepatectomy is generally recommended in patients with lesions ≥ 5 cm, because of increased risk of rupture as well as malignant degeneration[1,5]. The case we present, however, suggests a role of selective arterial embolization not only in the primary emergency situation, but also as a therapeutic tool to achieve necrosis of an arterially vascularized liver tumor.
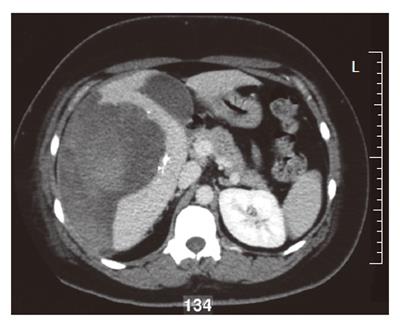
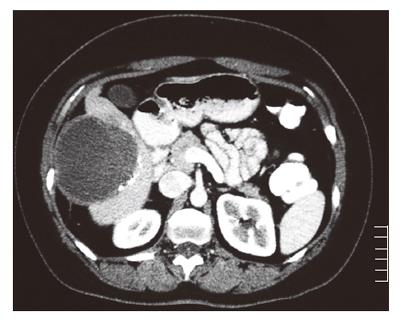
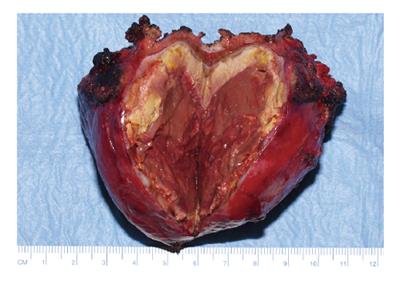
A 35-year old woman was referred to our hospital with a two-day history of right abdominal pain without any previous trauma. The patient had no medical history, her medication consisted of oral contraceptive treatment only (30 μg ethinylestradiol, 150 μg levonorgestrel, taken during eleven years). Despite initial fluid supplementation, her heart rate was elevated (110/min) with normal blood pressure. Further physical examination showed tenderness of the right upper abdomen. Hemoglobin levels were low (78 g/L). INR was 1.0, APTT 21.5 s, PTT 12.5 s and thrombocytes 213 × 109/L. Serum liver enzyme levels were elevated (ASAT 550 U/L, ALAT 607 U/L, Alk.Fos 75 U/L, γGT 109 U/L, LDH 758 U/L). She was transfused with 2 units of red blood cells. Computed tomography (CT) scan of the abdomen revealed a large subcapsular hematoma surrounding a tumorous mass in segment IV-VII of the liver, as well as intraperitoneal fluid. Arteriography confirmed hemorrhage originating from the right hepatic artery, which was also responsible for the vascularization of the tumor. Subsequently, selective arterial embolization of the majority of right hepatic artery branches was performed in order to stop the bleeding using both polyvinyl alcohol (PVA) particles and a mixture of enbucrilate (histoacryl) and iodized poppy-seed oil (lipiodol). Contrasr-enhanced CT scan 6 d after embolization clearly showed a circumscribed tumorous mass (∅ 7 cm) in segment V of the liver, most likely a hepatocellular adenoma (Figure 1). Further hospital stay was unremarkable and the patient was discharged 11 d after admission. Following our department’s protocol on liver adenomas with a diameter ≥ 5 cm, elective resection of segment V was performed six months later. Preoperative contrast-enhanced CT scan showed the remains of both the hematoma and the known circumscribed mass (Figure 2). Arterial perfusion of segment V of the liver was absent whereas portal perfusion was intact. The resected tumor consisted completely of necrotic material (Figure 3). No clear tumor classification could be made by histological examination due to absence of tumorous material, but the macroscopic signs of necrosis were confirmed. Tumor markers (CEA, CA 19.9 and α-foetoprotein) were in the normal range. The patient was discharged without complications, and subsequent follow-up until 22 mo after resection did not reveal any new lesions in the liver. She was advised to refrain from oral contraceptive medication.
The increased chance of rupture of large hepatocellular adenomas makes the presence of such a tumour a considerable potential hazard. Management of this condition still needs improvement. The presence of a ruptured hepatocellular adenoma cannot be proven by CT scan alone, but is strongly suggested by our patient’s clinical presentation. Although hepatocellular carcinoma and focal nodular hyperplasia lesions have also been described as causes of liver hemorrhage[7], their presence is unlikely in this patient. The presence of liver adenomas in young women is associated with the use of oral contraceptives. In this case no specific histologic tumor classification could be made, since cessation of arterial blood flow by embolization caused necrosis of the tumor. This corresponds with the fact that hepatocellular adenomas are only vascularized arterially. It has to be noted that regression of liver adenomas is possible not only after ischemia, but also after hormone withdrawal[8], hemodialysis[9] and dietary therapy for glycogen storage disease[10].
Embolization is safe and successful in stopping the hemorrhage. The necrosis of the tumor may point to a future role of selective arterial embolization in the management of either bleeding or non-bleeding hepatocellular adenomas. Furthermore, this case argues in favour of conservative follow-up after embolization, regardless of the initial size of the adenoma. This would further limit the indications for surgery in a selected group of patients, resulting in reduction of patient morbidity and mortality.
S- Editor Wang GP L- Editor Wang XL E- Editor Bai SH
| 1. | Ault GT, Wren SM, Ralls PW, Reynolds TB, Stain SC. Selective management of hepatic adenomas. Am Surg. 1996;62:825-829. [PubMed] |
| 2. | Rooks JB, Ory HW, Ishak KG, Strauss LT, Greenspan JR, Hill AP, Tyler CW Jr. Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA. 1979;242:644-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 286] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 3. | Shortell CK, Schwartz SI. Hepatic adenoma and focal nodular hyperplasia. Surg Gynecol Obstet. 1991;173:426-431. [PubMed] |
| 4. | Leese T, Farges O, Bismuth H. Liver cell adenomas. A 12-year surgical experience from a specialist hepato-biliary unit. Ann Surg. 1988;208:558-564. [RCA] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 191] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Terkivatan T, de Wilt JH, de Man RA, van Rijn RR, Zondervan PE, Tilanus HW, IJzermans JN. Indications and long-term outcome of treatment for benign hepatic tumors: a critical appraisal. Arch Surg. 2001;136:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Wheeler PG, Melia W, Dubbins P, Jones B, Nunnerley H, Johnson P, Williams R. Non-operative arterial embolisation in primary liver tumours. Br Med J. 1979;2:242-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Kleespies A, Settmacher U, Neuhaus P. [Spontaneous rupture of hepatic focal nodular hyperplasia--a rare cause of acute intraabdominal bleedingf]. Zentralbl Chir. 2002;127:326-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Aseni P, Sansalone CV, Sammartino C, Benedetto FD, Carrafiello G, Giacomoni A, Osio C, Vertemati M, Forti D. Rapid disappearance of hepatic adenoma after contraceptive withdrawal. J Clin Gastroenterol. 2001;33:234-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Iijima H, Moriwaki Y, Yamamoto T, Takahashi S, Nishigami T, Hada T. Spontaneous regression of hepatic adenoma in a patient with glycogen storage disease type I after hemodialysis: ultrasonographic and CT findings. Intern Med. 2001;40:891-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Parker P, Burr I, Slonim A, Ghishan FK, Greene H. Regression of hepatic adenomas in type Ia glycogen storage disease with dietary therapy. Gastroenterology. 1981;81:534-536. [PubMed] |