Published online Oct 7, 2006. doi: 10.3748/wjg.v12.i37.6050
Revised: July 23, 2005
Accepted: August 20, 2005
Published online: October 7, 2006
AIM: To examine the expression of metalloproteinase-1 (MMP-1) and tissue inhibitor of metalloproteinase-1 (TIMP-1) in the colonic mucosa of patients with ulcerative colitis (UC).
METHODS: Reverse transcription-polymerase chain reaction (RT-PCR) and immunohistochemistry were used to study the expression of MMP-1 and TIMP-1 at both mRNA and protein levels in patients with UC and controls. The relationship between MMP-1 mRNA, TIMP-1 mRNA, MMP-1 mRNA/TIMP-1 mRNA ratio and the severity of clinical symptoms of the patients with UC were also analyzed.
RESULTS: The expression of MMP-1 mRNA and TIMP-1 mRNA in the ulcerated and inflamed colonic mucosa was significantly higher than that in the non-inflamed colonic mucosa (P < 0.001), but there was no statistically significant difference in the non-inflamed colonic mucosa of UC patients and normal controls (P > 0.05). The mRNA expression of MMP-1 and TIMP-1 in ulcerated colonic mucosa of UC patients was increased by 80-fold and 2.2-fold, respectively when compared with the normal controls. In the inflamed colonic mucosa, the increase was 30-fold and 1.6-fold, respectively. Immunohistochemical analysis showed that among the ulcerated, inflamed, and non-inflamed colonic mucosae of UC patients and the normal controls, the positive rate of MMP-1 expression was 87%, 87%, 40% and 35% respectively, and the positive rate of TIMP-1 expression was 89%, 89%, 80% and 75%, respectively. Furthermore, the expression of MMP-1 mRNA, TIMP-1 mRNA and the MMP-1 mRNA/ TIMP-1 mRNA ratio were correlated with the severity of clinical symptoms (P <0.05).
CONCLUSION: Excessive expression of MMP-1 in the diseased colonic mucosa causes excessive hydrolysis of the extracellular matrix (ECM) and ulceration in UC patients. MMP-1 mRNA, TIMP-1 mRNA and MMP-1 mRNA/TIMP-1 mRNA ratio can be used as biomarkers to judge the severity of clinical symptoms in patients with UC. Exogenous TIMP-1 or MMP-1 inhibitor therapy is a novel treatment for patients with UC.
- Citation: Wang YD, Yan PY. Expression of matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 in ulcerative colitis. World J Gastroenterol 2006; 12(37): 6050-6053
- URL: https://www.wjgnet.com/1007-9327/full/v12/i37/6050.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i37.6050
Ulcerative colitis (UC) is a chronic, non-specific inflammatory disease of the colonic mucosa with unknown etiology and pathogenesis. Pathologically, it is characterized by ulceration in the mucosal and submucosal areas, and degradation of extracellular matrix (ECM) is one of the major events during this process[1]. Matrix metalloproteinase-1 (MMP-1) produced by cytokine-activated interstitial cells is one of the most important enzymes in degrading ECM, and the activity of MMP-1 is controlled by its natural inhibitor, tissue inhibitor of metalloproteinase (TIMP-1)[2]. Therefore, in this study we measured MMP-1 and TIMP-1 transcripts and their proteins by using reverse transcription-polymerase chain reaction (RT-PCR) and immunohistochemistry to explore their possible role in patients with UC.
Thirty patients with UC confirmed by colonoscopy and biopsy were enrolled in the study. Among these patients, 21 were males and 9 were females with their age ranged from 18 to 76 years and averaged 48 years. Samples were taken from the ulcerated, inflamed and non-inflamed areas of the colonic mucosa during colonoscopy. There were 3 patients with pan-colonic lesions, 2 with hemi-colonic lesions, 13 with recto-sigmoidal lesions, and 12 with rectal lesions. Based on the clinical manifestations, 2 patients were classified into severe type, 18 into moderate type, and 10 into mild type. Meanwhile, 20 normal subjects were selected as controls; 14 of them were males and 6 were females with their age ranged from 27 to 65 years and averaged 46 years. Biopsy samples were immediately snap frozen in liquid nitrogen and stored at -80°C for RT-PCR. Separate biopsy samples were fixed in formalin and embedded in paraffin for immunohistochemistry.
Total RNA was extracted from the frozen samples using the RNA isolation kit (Invitrogen Company) following the manufacturer’s instructions. Five μL of the extracted RNA was run on 1% agarose gel electrophoresis to identify the extracted products.
RT-PCR was performed using the TaKaRa RNA PCR kit 3.0 (AMV) (supplied by Dalian Baosheng Biotechnology Company) following the manufacturer’s instructions. Primer sequences[3] used were as follows: MMP-1: sense: 5’-ATGCGAACAAATCCCTTCTACC-3’, antisense: 5’-TTTCCTCAGAAAGAGCAGCATCG-3’; TIMP-1: sense: 5’-GGACACCAGAAGTCAACCAGCC-3’, antisense: 5’-CGTCCACAAGCAATGAGTCC-3’. Primers forβ-actin were used as the internal control: sense: 5’-CCTTCCTGGGCATGGAGTCCTG-3’, antisense: 5’-GGAGCAATGATCTTGATCTTC-3’. Reverse transcription was carried out at 30°C for 10 min, at 42°C for 30 min, at 99°C for 5 min, and at 5°C for 5 min. PCR was performed as follows: initial denaturation at 94°C for 2 min, 35 amplification cycles at 94°C for 30 s, at 53°C for 30 s, at 72°C for 1 min, extension at 72°C for 10 min. Five μL PCR product was run on 2% agarose gel electrophoresis.
Sample sections were washed 3 times with PBS, 3 min each time after initial treatment. Primary antibodies, rabbit anti-human MMP-1 polyclonal antibody and TIMP-1 monoclonal antibody (Beijing Zhongshan Biology Company) were added and incubated at room temperature for 1.5 h, washed again and incubated with peroxidase-conjugated secondary antibody for 15 min and washed again. A brown product was developed in diaminobenzidine (DAB) for 10 min.
Bio-imaging system (PALL Company, USA) was employed to analyze the density of the bands of PCR products. MMP-1 mRNA and TIMP-1 mRNA were semi-quantitatively expressed by the ratios between MMP-1, TIMP-1 and β-actin OD values. All values were expressed as mean ± SD.
Results of immunohistochemistry were scored according to the degree of staining and percentage of positive cells as no staining: 0 point, mild staining: 1 point, moderate staining: 2 points, heavy staining: 3 points; positive cells ≤ 5%: 0 point, 6%-30%: 1 point, 31%-70%: 2 points, 71%-100%: 3 points. Final score was determined by combining points obtained from the above two scoring systems: 0-1 point: (-), 2 points: (+), 3-4 points: (++), 5-6 points: (+++).
Student-Neuman-Keuls test was used to compare MMP-1 mRNA and TIMP-1 mRNA in different colon biopsy samples. Positive rates were analyzed by χ2 test and Spearman correlation analysis was used to study the relationship between MMP-1 mRNA, TIMP-1 mRNA, MMP-1 mRNA/TIMP-1 mRNA ratio and the severity of clinical symptoms. P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 10.0 for Windows.
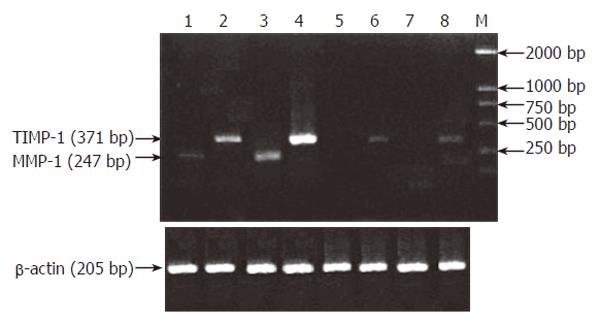
Expression of MMP-1 mRNA in the ulcerated and inflamed areas of the colon was significantly higher than that in the non-inflamed areas of the colon of UC patients and normal controls (P < 0.001). It was 80-fold higher in the ulcerated area and 30-fold higher in the inflamed area when compared with the normal controls, but there was no statistically significant difference in MMP-1 mRNA expression between UC patients and normal controls (P > 0.05, Figure 1, Table 1).
Expression of TIMP-1 mRNA in the ulcerated and inflamed area of colon was significantly higher than that in the non-inflamed area of colon of UC patients and normal controls (P < 0.001). It was 2.2-fold higher in the ulcerated area and 1.6-fold higher in the inflamed area when compared with the normal controls, but there was no statistically significant difference in TIMP-1 mRNA expression between UC patients and normal controls (Figure 1, Table 2).
Expression of MMP-1 mRNA was significantly related to the expression of TIMP-1 mRNA in the tissues of UC patients, the relating coefficient was 0.801 (P < 0.001). Based on clinical manifestations, only 2 patients were classified into severe type, so correlation analysis was performed between moderate group (18 patients) and mild group (10 patients). The results showed that MMP-1 mRNA, TIMP-1 mRNA and MMP-1 mRNA/TIMP-1 mRNA ratio were all significantly correlated with the severity of the clinical symptoms, and the relating coefficient was 0.411, 0.328 and 0.552, respectively.
Positive expression rates of MMP-1 in the ulcerated and inflamed areas of the colon were significantly higher than those in the non-inflamed areas of the colon of UC patients and normal controls (P < 0.05), but there was no statistically significant difference in non-inflamed areas between UC patients and normal controls (Table 3).
The intensity of MMP-1 expression in the ulcerated and inflamed area of colon was significantly higher than that in the non-inflamed area of colon of UC patients and normal controls (P < 0.001), but there was no statistically significant difference in the non-inflamed area between UC patients and normal controls (Table 4).
Positive expression rates of TIMP-1 in the ulcerated and inflamed area of colon were not statistically different when compared with those in the non-inflamed area of colon of UC patients and normal controls (Table 5).
| Samples | Positive cases | Negative cases | Total cases | Positive rate (%) | P value |
| Ulcerated area | 26 | 4 | 30 | 89 | > 0.05 |
| Inflamed area | 26 | 4 | 30 | 89 | > 0.05 |
| Non-inflamed area | 24 | 6 | 30 | 80 | > 0.05 |
| Normal controls | 15 | 5 | 20 | 75 |
The intensity of TIMP-1 expression in the ulcerated and inflamed area of colon was significantly higher than that in the non-inflamed area of colon of UC patients and normal controls (P < 0.001). The results also showed that the intensity of TIMP-1 expression in the ulcerated area was significantly greater than that in the inflamed area (P < 0.05), but there was no statistically significant difference in the non-inflamed area between UC patients and normal controls (Table 6).
UC is a chronic and non-specific inflammatory disease of the colon and affects mainly the colonic mucosa and submucosa. Pathologically, it is characterized by ulceration in the mucosa and submucosa and degradation of ECM is involved in this process. In this study, we separately utilized RT-PCR and immunohistochemistry to detect the expression of MMP-1 and TIMP-1 at both mRNA and protein levels. The expression of MMP-1 mRNA and TIMP-1 mRNA in ulcerated and inflamed areas of colon was significantly higher than that in the non-inflamed area of colon of UC patients and normal controls, the same results were also obtained at the protein level, suggesting that expression of MMP-1 and TIMP-1 is correlated to UC severity at both transcription and protein levels[4]. MMP-1, also termed interstitial collagenase, is able to degrade the spiral structure of collagen types I, II, III and X, making them more sensitive to the hydrolysis of gelatinase, and thus, play an important role in the degradation of ECM[5]. RT-PCR showed that the expression of MMP-1 mRNA was greatly increased in the ulcerated and inflamed areas of the colon of UC patients with the ulcerated area being more profound, suggesting that MMP-1 is related to the mucosal damage[6]. Arihiro et al[7] showed that MMP-1 has something to do with the initial steps of ulceration in UC. von Lampe et al[3] also found that the expression of MMP-1 is increased by 230-fold in the colonic mucosa of patients with UC compared with normal controls. Meanwhile, our immunohistochemical results showed that MMP-1 protein was significantly increased in the ulcerated and inflamed areas of UC patients. Furthermore, MMP-1 protein was mainly expressed in the interstitial cells. This is similar to the result of von Lampe et al[3] who identified that these interstitial cells are macrophages. Mckaig et al[8] identified that myofibroblasts are also MMP-1 producing interstitial cells in the colon of patients with UC.
TIMPs are natural inhibitors of MMPs and exert important regulating functions on MMPs. TIMP-1 mainly inhibits the activity of MMP-1, -3 and -9. Our results showed that the expression of TIMP-1 mRNA in the ulcerated and inflamed areas was significantly higher than that in the normal controls, suggesting that the increased expression of MMP-1 up-regulates TIMP-1, leading to an imbalanced state of MMP-1 mRNA/TIMP-1 mRNA ratio, or in other terms, the increased TIMP-1 mRNA was not able to counteract the increased MMP-1 mRNA, resulting in over degradation of ECM in UC. The similar result was also obtained when TIMP-1 protein was measured. Both MMP-1 and TIMP-1 were expressed in the interstitial cells, indicating that MMP-1 and TIMP-1 come from the same cells[2]. Immuno-electron microscopy reveals that MMP-1 and TIMP-1 are localized in the rough endoplasmic reticulum of the activated myofibroblasts and smooth muscle cells of the blood vessels[7], suggesting that MMP-1 and TIMP-1 have something to do with the formation of new blood vessels.
Based on clinical manifestations, only 2 UC patients were classified as severe type in our study. We analyzed the relationship between MMP-1 mRNA and TIMP-1 mRNA expression and the severity of the disease. Our results showed that expression of MMP-1 mRNA and TIMP-1 mRNA in patients with moderate UC was significantly higher than that in patients with mild UC. MMP-1 mRNA, TIMP-1 mRNA and MMP-1 mRNA/TIMP-1 mRNA ratio all were closely related to the severity of the clinical manifestations. Therefore, it is concluded preliminarily that MMP-1 mRNA, TIMP-1 mRNA and MMP-1 mRNA/TIMP-1 mRNA ratio can be used as the parameters in judging the clinical severity of patients with UC. von Lampe et al[3] found that MMP-1 mRNA expression is correlated with the pathological staging of inflammation. Wiercinska-Drapalo et al[9] found that serum TIMP-1 level is positively correlated with the extent of endoscopic mucosal injury, clinical severity and concentration of C reactive protein in UC patients.
In conclusion, excessive expression of MMP-1 in the diseased colon mucosa of UC patients causes excessive hydrolysis of the ECM and ulceration. MMP-1 mRNA, TIMP-1 mRNA and MMP-1 mRNA/TIMP-1 mRNA ratio can be used as biomarkers to judge the severity of clinical symptoms in patients with UC. Exogenous TIMP-1 or MMP-1 inhibitor therapy is a novel treatment for patients with UC.
S- Editor Wang J L- Editor Wang XL E- Editor Bi L
| 1. | Pender SL, Tickle SP, Docherty AJ, Howie D, Wathen NC, MacDonald TT. A major role for matrix metalloproteinases in T cell injury in the gut. J Immunol. 1997;158:1582-1590. [PubMed] |
| 2. | Woessner JF Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145-2154. [PubMed] |
| 3. | von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut. 2000;47:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 290] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 4. | Wang H, Keiser JA. Vascular endothelial growth factor upregulates the expression of matrix metalloproteinases in vascular smooth muscle cells: role of flt-1. Circ Res. 1998;83:832-840. [RCA] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 308] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Bailey CJ, Hembry RM, Alexander A, Irving MH, Grant ME, Shuttleworth CA. Distribution of the matrix metalloproteinases stromelysin, gelatinases A and B, and collagenase in Crohn's disease and normal intestine. J Clin Pathol. 1994;47:113-116. [RCA] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 110] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Di Sebastiano P, di Mola FF, Artese L, Rossi C, Mascetta G, Pernthaler H, Innocenti P. Beneficial effects of Batimastat (BB-94), a matrix metalloproteinase inhibitor, in rat experimental colitis. Digestion. 2001;63:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Arihiro S, Ohtani H, Hiwatashi N, Torii A, Sorsa T, Nagura H. Vascular smooth muscle cells and pericytes express MMP-1, MMP-9, TIMP-1 and type I procollagen in inflammatory bowel disease. Histopathology. 2001;39:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | McKaig BC, McWilliams D, Watson SA, Mahida YR. Expression and regulation of tissue inhibitor of metalloproteinase-1 and matrix metalloproteinases by intestinal myofibroblasts in inflammatory bowel disease. Am J Pathol. 2003;162:1355-1360. [RCA] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 156] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Wiercinska-Drapalo A, Jaroszewicz J, Flisiak R, Prokopowicz D. Plasma matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 as biomarkers of ulcerative colitis activity. World J Gastroenterol. 2003;9:2843-2845. [PubMed] |