Published online Sep 28, 2006. doi: 10.3748/wjg.v12.i36.5780
Revised: July 7, 2006
Accepted: July 17, 2006
Published online: September 28, 2006
AIM: To investigate the inhibitory effect of As2O3 on angiogenesis of tumor and expression of vascular endothelial growth factor (VEGF) in tumor cells in vivo and in vitro.
METHODS: The solid tumor model was formed in nude mice with the gastric cancer cell line SGC-7901. The animals were randomly divided into three groups. As2O3 was injected into the arsenic-treated groups (2.5 mg/kg and 5 mg/kg) and the same volume of saline solution was injected into the control group. Microvessel density (MVD) and expression of VEGF were detected with immunofluorescence laser confocal technology. Further expression of VEGF protein and VEGF mRNA was measured with Western bloting and fluorescence quantitative RT- PCR in SGC-7901 cells treated with As2O3.
RESULTS: In nude mice, after treatment with 5 mg/kg and 2.5 mg/kg As2O3 respectively, about 50% and 30% tumor growth inhibition were observed correspondingly (P < 0.05, P < 0.05). Decrease in MVD appeared in As2O3-treated tumors compared with control group (P < 0.001, P < 0.001). MVD in tumors was significantly lower in 5 mg/kg group than in 2.5 mg/kg group (P < 0.01). The fluorescence intensity levels of VEGF in tumor cells were significantly lowered in the arsenic-treated groups (P < 0.01, P < 0.01). The fluorescence intensity level of VEGF in 5 mg/kg group was lower than that in 2.5 mg/kg group (P < 0.01). In vitro, the expression of VEGF protein decreased in dose- and time-dependent manner after the treatment with As2O3, but in VEGF mRNA no significant difference was found between the control group and the treated groups.
CONCLUSION: As2O3 can inhibit solid tumor growth by inhibiting the formation of new blood vessels. One of the mechanisms is that As2O3 can inhibit VEGF protein expression.
- Citation: Xiao YF, Liu SX, Wu DD, Chen X, Ren LF. Inhibitory effect of arsenic trioxide on angiogenesis and expression of vascular endothelial growth factor in gastric cancer. World J Gastroenterol 2006; 12(36): 5780-5786
- URL: https://www.wjgnet.com/1007-9327/full/v12/i36/5780.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i36.5780
Arsenic has been used since ancient times as a therapeutic agent. However, until recently its use in modern medicine has been restricted to the treatment of a limited number of parastic infections. Since the early 1990s, arsenic trioxide (As2O3) has been used successfully in the treatment of patients with relapsed and refractory acute promyelocytic leukemia (APL)[1,2]. As2O3 has been shown to cause degradation of PML-RAR alpha, promoting differentiation[3,4]. However, degradation of PML-RAR alpha may not be wholly responsible for the great sensitivity of APL cells to As2O3. Other investigations subsequently found that As2O3 can induce apoptosis of APL cells, other malignant myeloid, lymphoid, and megakaryocytic cells.
The mechanism by which arsenic exerts effect of inhibiting tumor still remains uncertain. Investigations of arsenic have found that its efficacy is dependent upon a number of mechanisms. Arsenic may affect numerous intracellular signal transduction pathways and causes many alterations in cellular function[5-8]. Recently, it has been reported that As2O3 can decrease microvessel density and inhibit angiogenesis in solid tumors[9-11], but its pathway is not clear.
Angiogenesis is an important factor in the progression and enlargement of solid neoplasms and is in close relation to invasion and metastases[12-14]. Angiogenesis can be divided into a series of temporally regulated responses, including protease induction, endothelial cell migration, proliferation and differentiation[15]. This process begins with the production and release of angiogenic factors by endothelial cells, tumor cells and matrix cells. Vascular endothelial growth factor (VEGF) produced by tumor cells is a potent angiogenic factor, and it binds to endothelial cell surface receptors and activates various functions of the cells[16-19]. Therefore, angiogenesis can be reflected by the expression of VEGF.
In the present report, we investigated the inhibitory effect of As2O3 on angiogenesis of tumor and expression of VEGF in tumor cells in vivo and in vitro. The solid tumor model was formed in nude mice with the gastric cancer cell line SGC-7901. Microvessel density (MVD) and expression of VEGF were detected by immunofluorescence laser confocal technology. In vitro we measured further expression of VEGF protein and VEGF mRNA with Western blots and real-time fluorescence quantitative RT-PCR.
Thirty 5-wk-old male BALB/C-nu/nu mice weighing 19-21 g were purchased from Shanghai Institute for Biological Sciences, Chinese Academy of Sciences, Shanghai, China. Mice were acclimatized at the Animal Laboratory Centre of the Fourth Military University for 1 wk before receiving injections of cancer cells. The mice were kept in a laminar-filtered airflow cabinet under pathogen-free conditions with a constant temperature of 22 ± 2°C, relative humidity of 55% ± 5% and 12-h dark/light cycles. All experiments were carried out according to the guidelines of the Laboratory Protocol of Animal Handling of the Fourth Military Medical University. Human gastric cancer cell line, SGC-7901, was purchased from the Animal Laboratory Centre of the Fourth Military Medical University. SGC-7901 cells were cultured in RPMI 1640 medium supplemented with 100 mL/L fetal bovine serum (FBS) at 37°C in a 50 mL/L CO2 incubator.
Arsenious acid [H3As2O3 (As2O3 + 3H2O ↔ H3As2O3)] was supplied by Yida Pharmaceutical Co, Harbin, China. VEGF expression in tumor xenografts was examined by a TCS SP2 laser confocal microscope made by LEICA Cc, Germany. VEGF mRNA was examined by real time system ABI7000 (Applied Biosystems, Foster City, CA, USA).
Thirty mice received sc injection into the right flanks with a 200 μL cell suspension containing 2 × 107 SGC-7901 cells. After 10 d, when established tumors of 0.2-0.3 cm3 diameter were detected, drug administration was started. The animals were randomly divided into three groups consisting of 10 animals in each. Arsenious acid diluted with 9 g/L saline solution was injected into the peritoneal cavum every day to the two treatment groups (2.5 mg As2O3/kg and 5 mg As2O3/kg in 0.2 mL) and the same volume of 9 g/L saline solution was injected to the control group. After 10 d of treatment, the three groups of mice were sacrificed and the tumor masses were removed. After the weights of tumor mass were measured, the tumor mass was fixed in 40 g/L paraformaldehyde and frozen sections prepared with a cryostat (HM505E Cryomicrom, Germany) for immunofluorescence analysis. The liver and kidneys were prepared for paraffin sections routinely for pathological examination.
Tumor growth inhibition (TGI) was used to assess the therapeutic efficacy against xenografts. The tumor weight was to calculate TGI, that is, TGI (%) = (1-Wt/Wc)×100%, where Wt is the mean tumor weight of the arsenic- treated group, Wc is the mean tumor weight of the control group. Tumor growth inhibition was expressed by tumor volumes that were measured before treatment and 6 and 11 d after treatment respectively. Tumor volume expressed as cm3 is calculated using the formula: tumor volume = 1/2ab, where a is the long axis, b is the short axis. TGI (%) = (1 - Vt/Vc) × 100%, where Vt is the mean tumor volume of the arsenic-treated group, Vc is the mean tumor volume of the control group.
CD31 antigen was used as an immunofluorescence marker of vessel endothelial cells. For the immunofluorescent staining of CD31 and VEGF, the frozen sections were kept at room temperature for 30 min, incubated in distilled water for 5 min and in PBS for 5 min, permeabilized in 1 g/L Triton-X-100 for 10 min, washed with PBS (5 min × 3), blocked with 100 mL/L sheep serum (Sgima) at 37°C for 20 min, incubated in the primary antibody, rat anti-mouse CD31 (Biolegend, USA) or rabbit anti-human VEGF polyclonal antibody (Labvision, USA) at 4°C overnight, washed with PBS (5 min × 3), incubated in the secondary antibody (goat anti-rat IgG conjugated TRITC or sheep anti-rabbit IgG conjugated FITC, Sigma) for 1h at 37°C, washed with PBS (10 min × 3) and then examined under a TCS SP2 laser confocal microscope.
For each group, several field images of VEGF in each tumor tissue section were captured under confocal microscope, fluorescence intensity of each section in the confocal fluorescence images was measured using the Leica Confocal analysis system. The mean fluorescence intensity in each section was then calculated.
Microvessel density (MVD) was determined under a confocal microscope in the area of tumor tissue sections. The microvessels were carefully counted on 20 fields (×400). The mean and SE were expressed as the number of microvessels identified within the area.
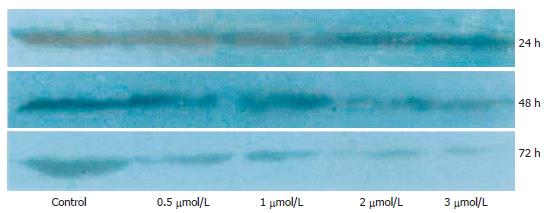
SGC-7901 cells were routinely grown in plastic tissue culture flasks in RPMI 1640 medium with 100 mL/L FBS, 100 KU/L penicillin and 100 mg/L streptomycin and were cultured at 37°C in a 50 mL/L CO2 humidified atmosphere. SGC-7901 cells in logarithmic growth phase were inoculated in culture medium, without adding (control group) and adding As2O3 according to different concentrations of below 0.5, 1, 2 and 3 μmol/L. The tumor cells were collected respectively at 24 h, 48 h and 72 h for Western blot of VEGF. The fluorescent quantitative RT-PCR of VEGF was analyzed in the tumor cells collected at 48 h.
After treatment with As2O3, SGC-7901 cells were washed with ice-cold PBS and dissolved with lysis buffer (1 mmol/L Na3VO4, 0.017 g/L approtinin, 2 mmol/L PMSF, 25 μmol/L P-N-P, 0.1 g/L leupeptin, 10 g/L NP-40, 0.15 mol/L NaCl, 0.02 mol/L Tris HCl, 0.1 g/L NAN3). The protein lysates were centrifuged at 12 000 r/min for 10 min at 4°C. The protein lysates were mixed with 5 × sample buffer, boiled for 10 min, and analyzed by 120 g/L sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After SDS-PAGE, the gel was transferred to a 0.45-μm pure nitrocellulose membrane. The membrane was blocked with 5 mL/L skim milk in TBS-Tween 20 for 1 h at room temperature, and reacted with mouse anti-human VEGF monoclonal antibody (Santa Cruz) at 4°C overnight. The membrane was then incubated with the secondary antibody (rabbit anti-mouse IgG). The protein content was visualized using HRP-conjugated secondary antibodies, followed by enhanced chemiluminescence (ECL).
RT-PCR for VEGF, total RNA was extracted from tumor cells using Trizol reagent according to the manufacturer’s protocol (Promega, Madison,WI,USA). RNA was reversely transcribed to cDNA by the kit (Promega, Madison, WI, USA). Prime and probe were designed with the assistance of Primer Express 2.0. MGB-TaqMan probe of VEGF (VEGF-FAM): 5’-ACCATGCAGATTATG-3’. For VEGF, the following primers were used: 5’-GCCCACTGAGGAGTCCAACA-3’, 5’TGGCCTTGGTGAGGTTTGAT-3′. MGB-TaqMan probe of β-actin (HBA-TAMRA-FAM): 5‘CCAGGGCGTGATGGTGGGCAT 3’. For β-actin the following primers were used: HBA-TAMRA-FP 5‘CCGTCTTCCCCTCCATCG 3’ HBA-TAMRA-RP 5‘GTCCCAGTTGGTGACGATGC 3’The primes and probes were synthesized by Shanghai GeneCore BioTechnologies Co., Ltd. All PCR reactions were performed on a real time system ABI7000 (Applied Biosystems, Foster City, CA, USA). The thermal cycle conditions comprised 2 min at 50°C, 10 min at 95°C, 30 s at 95°C and 30 s at 60°C. Each PCR run included five points of calibration curve (5 samples were obtained by tenfold serially dilutions of standard product, and the standard curve was established from the 5 samples for the determination of the relative quantity of genes), a non-template control, and the respective sample cDNA.
The data were represented as mean ± SD. The data was analyzed with statistical software of SPSS10.0. Multiple statistical comparisons were performed using ANOVA in a multivariate linear model. Student’s t test was used to assess differences between the treatment group and control group. P < 0.05 was considered statistically significant.
All nude mice developed tumor after 10 d in implantation of SGC-7901 cells. When drug administration was started, there was no significant difference in the tumor volume of the three groups (P > 0.05). The increase in tumor volume and weight in the three groups from d 0 to 11 after treatment is shown in Table 1. Tumor volume growth of 2.5 mg/kg or 5 mg/kg of the arsenic-treated group was reduced by 30.3% or 50.9% after 10 d administration of arsenic trioxide. Tumor weight growth was inhibited by 29.1% and 52.2% respectively in the 2.5 mg/kg and 5 mg/kg arsenic -treated group. There were statistical differences in the tumor volumes and weights in the arsenic -treated group (2.5 mg/kg group and 5 mg/kg group) and control (P < 0.05). On the other hand, the tumor volumes and weights in the 5 mg/kg group were significantly less than that in 2.5 mg/kg group (P < 0.05). No obvious toxic effects attributable to As2O3 therapy were noted. The body weight of the mice was not affected. All of the 30 studied mice were alive at the end of treatment. HE staining of the nude mice’s liver and kidney did not reveal pathological changes.
Sections of tumors from the mice in all three treatment groups were stained for CD31 immunofluorescence to detect the number of endothelial cells (ECs) as a measure of tumor angiogenesis. A single microvessel was defined as any immunofluorescence stained endothelial cell distinguished from adjacent tumor cells and other connective tissue elements. Microvessel density as a parameter of angiogenic activity was decreased in tumors of experimental groups treated with 2.5 mg/kg (Figure 1B) and 5 mg/kg arsenic trioxide (Figure 1C). Microvessel density of arsenic-treated groups was significantly lower than that of the control group (Figure 1A). Significantly lower microvessel density was present in the 5 mg/kg group than that in the 2.5 mg/kg group. These studies demonstrated the decreased capillary density of the tumor with treatment of As2O3 (Table 2).
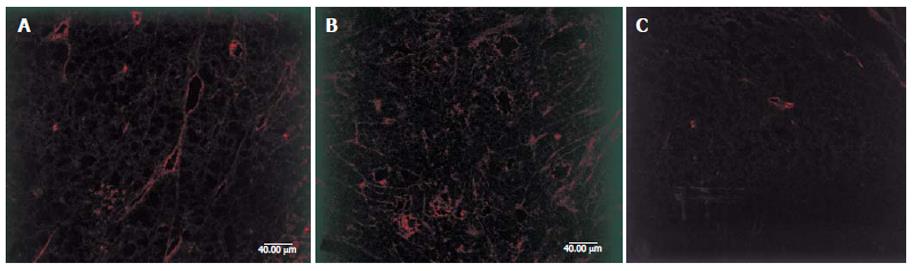
Expression of VEGF was confirmed by the presence of fluorescence-stained cytoplasm in the tumor cells. It is shown in Figure 2, strong immunoreactivity to VEGF was found in all the SGC-7901 tumor xenografts of the control group (Figure 2A). The weaker fluorescence intensity expression was observed in SGC-7901 tumor of the groups treated with 2.5 and 5 mg/kg arsenic trioxide (Figure 2B and C). The fluorescence intensity levels of VEGF in tumor cells were significantly lower in the arsenic -treated groups than in control group (P < 0.01). The fluorescence intensity level of VEGF in 5mg/kg group was lower than that in 2.5 mg/kg (P < 0.01, Table 2).
To further analyze the effect of arsenic trioxide on VEGF expression in tumor, we assessed VEGF expressions of SGC-7901 cells in vitro using Western blotting and fluorescent quantitative RT-PCR. Western blot analysis revealed that the expression of VEGF protein decreased in a dose- and time-dependent manner after treatment with As2O3 (Figure 3). But VEGF mRNA expression was not in agreement with the VEGF protein expression results, VEGF mRNA data did not reveal a significant difference between the control group and the treatment groups (Table 3, Figures 4 and 5).
| As2O3(μmol/L) | VEGF mRNA | β-actin mRNA | VEGF mRNA/β-actin mRNA |
| 0 | 1.28 × 106 | 3.72 × 107 | 3.44 × 10-2 |
| 0.5 | 2.34 × 104 | 7.92 × 105 | 2.95 × 10-2 |
| 1.0 | 4.74 × 102 | 9.96 × 103 | 4.76 × 10-2 |
| 2.0 | 2.16 × 106 | 7.24 × 107 | 2.89 × 10-2 |
| 3.0 | 3.21 × 103 | 8.2 × 104 | 3.91 × 10-2 |
The development of a growing tumor requires an abundant blood supply. Angiogenesis is an important factor in the progression and enlargement of solid neoplasms and close relation to invasion and metastases[12-14]. Vascularisation within the primary tumor has a direct relation with the tumor growth rate. Inhibition of angiogenesis may lead to control of tumor growth and metastasis, therefore, antiangiogenesis is a promising therapeutic approach for treatment of cancer[20-22]. The results presented here indicated that the As2O3 is active against angiogenesis in vivo and in vitro. We examined the effect of As2O3 on angiogenesis in a human tumor model in nude mice. The result showed that subcutaneous injection of SGC-7901 cells yielded a 100% tumor uptake rate. About 50% and 30% SGC-7901 tumor growth inhibition was following treatment of As2O3 with 5 mg/kg and 2.5 mg/kg separately.
We measured the MVD in tumor tissues by labeled CD31, which marked endothelial cells of microvessels. Decrease of the MVD in tumor tissue was related to treatment of As2O3. A high degree of tumor angiogenesis was observed in mice without treatment of As2O3, but MVD was low in As2O3-treated tumors. There was a significant difference in MVD between the control group and treatment groups. MVD was significantly lower in the high dosage group with 5 mg/kg As2O3 than in the low dosage group with 2.5 mg/kg. Our observation was in agreement with the results of Shen[9]. This study demonstrated that As2O3 induced a dose-dependent decrease of MVD, accompanied by inhibition of tumor growth. These suggest that induction of angiogenesis is an important step in carcinogenesis and As2O3 inhibits tumor growth by, at least in part, decreasing intratumor MVD.
It has been reported that neovascularization is regulated by accelerators, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (FGF), hepatocyte growth factor (HGF), thymidine phosphorylase, endothelin, IL-4, and IL-8, and by inhibitors such as thrombospondin, endostatin, angiostatin, transforming growth factorβ1 (TGF-β1), tumor necrotic factor (TNF) and IL-6[23]. Of these factors, VEGF is involved very much in the proliferation and metastasis of various cancers. There is evidence that the degree of several surrogate angiogenic markers such as microvessel density or (VEGF) expression levels in primary tumor tissue can reflect the biological aggressiveness of tumors and provide prognostic information[18,23-25].
The VEGF gene family currently includes six members: VEGF-A (prototype VEGF), VEGF-B, VEGF-C, VEGF-D, VEGF-E and placenta growth factor. Of them, vascular VEGF, also known as vascular permeability factor, is a potent angiogenic cytokine that induces mitosis and also regulates permeability of endothelial cells. In addition, VEGF prolongs the survival of new blood vessels by inducing the expression of Bcl-2 protein. VEGF plays a crucial role in vasculogenesis and angiogenesis, and is closely related to invasion and metastases of tumors[26,27].
Several studies reported that the degree of VEGF expression in tumors correlated positively with the level of MVD, the degree of malignancy, stage of tumor, presence/absence of peritoneal dissemination and metastases, and correlated negatively with prognosis[12,23,28]. In the present study, high expression VEGF existed in tumor tissues without treatment of As2O3 which also showed an increase of MVD, suggesting that VEGF is most closely associated with induction and maintenance of the neovasculature in human gastric cancer. Since human gastric cancer SGC-7901 cells secrete high amounts of VEGF and develop in nude mice tumors whose growth is highly VEGF-dependent, they provide a good model to test the availability of molecules that inhibit VEGF bioactivity.
In recent years, it has been widely shown that VEGF activity is a key feature during tumor growth and angiogenesis, and that blocking of this signal transduction pathway may inhibit tumor progression. In vivo, VEGF acts as a potent mitogenic factor for endothelial cells and as blood vessel permeabilising agents[29]. Our in vivo study indicated that expression of VEGF was significantly reduced after As2O3 treatment, which demonstrated that As2O3 can inhibit VEGF expression. The formation of new blood vessels is inhibited due to suppression of tumor cell secretion of VEGF by As2O3.
We further explored the mechanism of the inhibitory action of As2O3 on VEGF in tumor cells in vitro. We measured expression of VEGF protein and VEGF mRNA in the SGC-7901 cell line with Western blots and real time RT-PCR separately. The results showed that As2O3 induced a dose-and time-dependent down-regulation of VEGF protein expression in the SGC-7901 cell line, but did not affect VEGF mRNA expression. The results reveal the effect of As2O3 on VEGF expression on the protein level rather than the mRNA level.
These studies have demonstrated that As2O3 can inhibit solid tumor growth in vivo, possibly by inhibiting the formation of new blood vessels. One of the mechanisms is that As2O3 can inhibit VEGF protein expression. These results may be useful in devising better strategies for cancer treatment.
S- Editor Pan BR L- Editor Ma JY E- Editor Bi L
| 1. | Wang ZY. Arsenic compounds as anticancer agents. Cancer Chemother Pharmacol. 2001;48 Suppl 1:S72-S76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354-3360. [PubMed] |
| 3. | Slack JL, Waxman S, Tricot G, Tallman MS, Bloomfield CD. Advances in the management of acute promyelocytic leukemia and other hematologic malignancies with arsenic trioxide. Oncologist. 2002;7 Suppl 1:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Davison K, Mann KK, Miller WH Jr. Arsenic trioxide: mechanisms of action. Semin Hematol. 2002;39:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Waxman S, Anderson KC. History of the development of arsenic derivatives in cancer therapy. Oncologist. 2001;6 Suppl 2:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 231] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Murgo AJ. Clinical trials of arsenic trioxide in hematologic and solid tumors: overview of the National Cancer Institute Cooperative Research and Development Studies. Oncologist. 2001;6 Suppl 2:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 148] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Miller WH Jr, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893-3903. [PubMed] |
| 8. | Miller WH Jr. Molecular targets of arsenic trioxide in malignant cells. Oncologist. 2002;7 Suppl 1:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Shen ZY, Shen J, Chen MH, Wu XY, Wu MH, Zeng Y. The inhibition of growth and angiogenesis in heterotransplanted esophageal carcinoma via intratumoral injection of arsenic trioxide. Oncol Rep. 2003;10:1869-1874. [PubMed] |
| 10. | Griffin RJ, Lee SH, Rood KL, Stewart MJ, Lyons JC, Lew YS, Park H, Song CW. Use of arsenic trioxide as an antivascular and thermosensitizing agent in solid tumors. Neoplasia. 2000;2:555-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Roboz GJ, Dias S, Lam G, Lane WJ, Soignet SL, Warrell RP Jr, Rafii S. Arsenic trioxide induces dose- and time-dependent apoptosis of endothelium and may exert an antileukemic effect via inhibition of angiogenesis. Blood. 2000;96:1525-1530. [PubMed] |
| 12. | Folkman J. Angiogenesis and apoptosis. Semin Cancer Biol. 2003;13:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 285] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 13. | Dekel Y, Koren R, Kugel V, Livne PM, Gal R. Significance of angiogenesis and microvascular invasion in renal cell carcinoma. Pathol Oncol Res. 2002;8:129-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Edel MJ, Harvey JM, Papadimitriou JM. Comparison of vascularity and angiogenesis in primary invasive mammary carcinomas and in their respective axillary lymph node metastases. Clin Exp Metastasis. 2000;18:695-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Klagsbrun M, Moses MA. Molecular angiogenesis. Chem Biol. 1999;6:R217-R224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 188] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | McCabe CJ, Boelaert K, Tannahill LA, Heaney AP, Stratford AL, Khaira JS, Hussain S, Sheppard MC, Franklyn JA, Gittoes NJ. Vascular endothelial growth factor, its receptor KDR/Flk-1, and pituitary tumor transforming gene in pituitary tumors. J Clin Endocrinol Metab. 2002;87:4238-4244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 136] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Zhang H, Wu J, Meng L, Shou CC. Expression of vascular endothelial growth factor and its receptors KDR and Flt-1 in gastric cancer cells. World J Gastroenterol. 2002;8:994-998. [PubMed] |
| 18. | Ghanem MA, van Steenbrugge GJ, Sudaryo MK, Mathoera RB, Nijman JM, van der Kwast TH. Expression and prognostic relevance of vascular endothelial growth factor (VEGF) and its receptor (FLT-1) in nephroblastoma. J Clin Pathol. 2003;56:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Ciardiello F, Bianco R, Caputo R, Caputo R, Damiano V, Troiani T, Melisi D, De Vita F, De Placido S, Bianco AR. Antitumor activity of ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor, in human cancer cells with acquired resistance to antiepidermal growth factor receptor therapy. Clin Cancer Res. 2004;10:784-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 265] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 20. | Jung YD, Kim MS, Shin BA, Chay KO, Ahn BW, Liu W, Bucana CD, Gallick GE, Ellis LM. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br J Cancer. 2001;84:844-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 230] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 21. | Sharma RA, Harris AL, Dalgleish AG, Steward WP, O'Byrne KJ. Angiogenesis as a biomarker and target in cancer chemoprevention. Lancet Oncol. 2001;2:726-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Ranieri G, Gasparini G. Angiogenesis and angiogenesis inhibitors: a new potential anticancer therapeutic strategy. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:241-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Yabushita H, Shimazu M, Noguchi M, Kishida T, Narumiya H, Sawaguchi K, Noguchi M. Vascular endothelial growth factor activating matrix metalloproteinase in ascitic fluid during peritoneal dissemination of ovarian cancer. Oncol Rep. 2003;10:89-95. [PubMed] |
| 24. | Ranieri G, Passantino L, Patruno R, Passantino G, Jirillo F, Catino A, Mattioli V, Gadaleta C, Ribatti D. The dog mast cell tumour as a model to study the relationship between angiogenesis, mast cell density and tumour malignancy. Oncol Rep. 2003;10:1189-1193. [PubMed] |
| 25. | Ranieri G, Coviello M, Chiriatti A, Stea B, Montemurro S, Quaranta M, Dittadi R, Paradiso A. Vascular endothelial growth factor assessment in different blood fractions of gastrointestinal cancer patients and healthy controls. Oncol Rep. 2004;11:435-439. [PubMed] |
| 26. | Poon RT, Lau C, Yu WC, Fan ST, Wong J. High serum levels of vascular endothelial growth factor predict poor response to transarterial chemoembolization in hepatocellular carcinoma: a prospective study. Oncol Rep. 2004;11:1077-1084. [PubMed] |
| 27. | Faviana P, Boldrini L, Spisni R, Berti P, Galleri D, Biondi R, Camacci T, Materazzi G, Pingitore R, Miccoli P. Neoangiogenesis in colon cancer: correlation between vascular density, vascular endothelial growth factor (VEGF) and p53 protein expression. Oncol Rep. 2002;9:617-620. [PubMed] |
| 28. | Cascinu S, Staccioli MP, Gasparini G, Giordani P, Catalano V, Ghiselli R, Rossi C, Baldelli AM, Graziano F, Saba V. Expression of vascular endothelial growth factor can predict event-free survival in stage II colon cancer. Clin Cancer Res. 2000;6:2803-2807. [PubMed] |
| 29. | Hamma-Kourbali Y, Starzec A, Vassy R, Martin A, Kraemer M, Perret G, Crépin M. Carboxymethyl benzylamide dextran inhibits angiogenesis and growth of VEGF-overexpressing human epidermoid carcinoma xenograft in nude mice. Br J Cancer. 2003;89:215-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |