Published online Sep 21, 2006. doi: 10.3748/wjg.v12.i35.5729
Revised: April 15, 2006
Accepted: April 27, 2006
Published online: September 21, 2006
Hepatocellular carcinoma (HCC) recurs with a reported frequency of 12%-18% after liver transplantation. Recurrence is associated with a mortality rate exceeding 75%. Approximately one-third of recurrences develop in the transplanted liver and are therefore amenable to local therapy. A variety of treatment modalities have been reported including resection, transarterial chemo-embolization (TACE), radiofrequency ablation (RFA), ethanol ablation, cryoablation, and external beam irradiation. Goals of treatment are tumor control and the minimization of toxic effect to functional parenchyma. Efficacy of treatment is mitigated by the need for ongoing immunosuppression. Yttrium-90 microspheres have been used as a treatment modality both for primary HCC and for pre-transplant management of HCC with promising results.
Twenty-two months after liver transplantation for hepatitis C cirrhosis complicated by HCC, a 42-year old man developed recurrence of HCC in his transplant allograft. Treatment of multiple right lobe lesions with anatomic resection and adjuvant chemotherapy was unsuccessful. Multifocal recurrence in the remaining liver allograft was treated with hepatic intra-arterial infusion of yttrium-90 microspheres (SIR-Spheres, Sirtex Medical Inc., Lake Forest, IL, USA). Efficacy was demonstrated by tumor necrosis on imaging and a decrease in alpha-fetoprotein (AFP) level. There were no adverse consequences of initial treatment.
- Citation: Rivera L, Giap H, Miller W, Fisher J, Hillebrand DJ, Marsh C, Schaffer RL. Hepatic intra-arterial infusion of yttrium-90 microspheres in the treatment of recurrent hepatocellular carcinoma after liver transplantation: A case report. World J Gastroenterol 2006; 12(35): 5729-5732
- URL: https://www.wjgnet.com/1007-9327/full/v12/i35/5729.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i35.5729
Strict criteria developed to select patients with hepatocellular carcinoma (HCC) for liver transplantation have increased the survival and decreased the recurrence of tumor after transplantation[1]. However, tumor recurrence and subsequent mortality continue to be observed. Tumor recurrence is reported to occur with a frequency of 12%-18%[2-4]. Seventy percent of recurrences are observed within the first year after transplantation[3]. Unfortunately, recurrence-related mortality is high and exceeds 75%[2]. Extra-hepatic recurrence is encountered most commonly, with an estimated incidence of 67%[2]. Recurrence isolated to the transplant liver is relatively infrequent, yet, it represents an opportunity for targeted treatment. Local therapies include resection, trans-arterial chemoembolization (TACE), radiofrequency ablation (RFA), ethanol ablation, cryoablation, and external beam irradiation. Treatment efficacy varies according to tumor characteristics such as location, size, and number. Efficacy also varies with the degree of underlying hepatic dysfunction. Hepatic arterial infusion of microspheres impregnated with the beta-radiation emitter yttrium-90 has been successfully employed in the palliative treatment of unresectable HCC and most recently to down-stage HCC to allow liver transplantation[5]. Results for the treatment of primary HCC reveal a 38%-65% partial response rate, and a median survival of 23 mo which is 2.6-4.7 times the length of median survival seen in historic controls[6].
The following case report describes a 42-year old man who underwent liver transplantation for hepatitis C cirrhosis with stage II HCC and 22 mo later developed recurrent HCC isolated to the liver allograft. When conventional treatment modalities failed, hepatic arterial infusion of yttrium-90 beads (SIR-Spheres, Sirtex Medical Inc., Lake Forest, IL, USA) was safely employed to treat the HCC recurrence in the liver allograft.
This hispanic man was diagnosed at 38 years of age with end-stage liver dysfunction secondary to hepatitis C infection (genotype 1a) and listed with UNOS for transplantation in 2001 (UNOS status 3, MELD score 12). Nine months after being listed, serial CT examination of the liver suggested a new 1.5 cm lesion in segment IV. Follow-up imaging five months later showed the lesion to have grown in size to 3 cm consistent with HCC (stage II, T2NxMx). AFP level at this time was 38.7 μg/L. He underwent percutaneous RFA of the lesion while awaiting transplantation with a slight decrease in the AFP level to 32.9 μg/L and no residual tumor on CT after treatment. He received the standard MELD exception score at the time of 29 for his stage II HCC. A deceased donor liver transplant became available 5 mo after receiving his exception score (3.5 mo after RFA and 23 mo after being originally listed for transplant). The donor was a 52-year old CMV-positive man. Standard caval interposition technique without veno-venous bypass was used and the total graft ischemic time was 7 h and 40 min. Induction immunotherapy consisted of 20 mg of basiliximab, 300 mg of azathioprine and 1 g of methylprednisolone. Contrary to the post-RFA CT scan from 10 wk earlier, pathologic evaluation of the hepatic explant was notable for the presence of three foci of HCC in the right lobe of the liver. The lesions measured 2.8 cm, 2.7 cm and 1.5 cm each, in maximum dimension. The largest was 60% necrotic as a result of RFA treatment. Each tumor was moderately or well differentiated and demonstrated no angiolymphatic invasion.
Following transplantation, steroid doses were tapered to 15 mg/d by postoperative day seven. Azathioprine was discontinued on postoperative day seven and maintenance immunosuppression with tacrolimus was begun. His first allograft biopsy three weeks after transplant revealed only mild ischemia-reperfusion changes and no evidence of rejection or recurrent HCV infection. The tacrolimus dose was adjusted to achieve a trough of 10-15 μg/L for the first three months after transplant. After three months, transaminases were elevated and a liver biopsy done at this time revealed recurrent HCV with grade 1 (0-4) inflammation and steatosis without fibrosis (stage 0, 0-4). Prednisone dose was tapered and subsequently discontinued. Tacrolimus doses were further decreased to achieve a target trough level of 6-10 μg/L. A one-year liver biopsy, however, demonstrated progression to fibrosis (stage 2, 0-4). Transaminases remained mildly elevated and new hyperbilirubinemia (total bilirubin 29.1 μmol/L) was also noted. Quantitative HCV RNA testing revealed high-level viremia with viral tiers of 5.8 GU/L. HCV treatment with pegylated interferon and ribavirin was begun. After a 15 wk course of interferon-based anti-viral therapy, he had only a transient decrease in transaminases and bilirubin with persistently high HCV RNA levels. HCV treatment was discontinued.
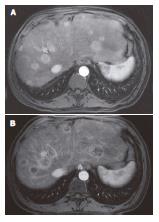
HCC surveillance with serial imaging and AFP levels remained negative for the first 18 mo following transplantation. An elevated AFP level of 16 μg/L was noted at 22 mo post transplant. A CT scan of the liver revealed a 3 cm nodule in segment VI not seen on the imaging five months earlier. Recurrent HCC was confirmed by needle biopsy, bone scan and chest CT were negative for distant metastatic disease. The patient was offered a segmental resection of the tumor and underwent laparotomy. No extra-hepatic tumor was identified. Intraoperative ultrasound revealed two additional 1 cm lesions in segments III and IV. These and the initial segment VI lesion were segmentally resected. The patient’s post-operative course was uncomplicated. Post-operative AFP level decreased to 7 μg/L. One month after surgery, maintenance immunotherapy was switched from tacrolimus monotherapy to sirolimus monotherapy with target trough levels of 5-8 μg/L and adjuvant chemotherapy with systemic doxorubicin (200 mg/m2 over 20 wk) was begun. Chemotherapy was complicated by the development of diabetes mellitus and anemia. AFP level increased to 820 μg/L despite chemotherapy. A CT scan done 3 mo into his course of doxorubicin showed vague hyper-enhancement in segment VIII and magnetic resonance imaging (MRI) scan of the liver obtained at the completion of treatment revealed uniformly enhancing, multifocal nodules consistent with HCC in the remaining liver allograft with the largest nodule measuring 3.8 cm (Figure 1A).
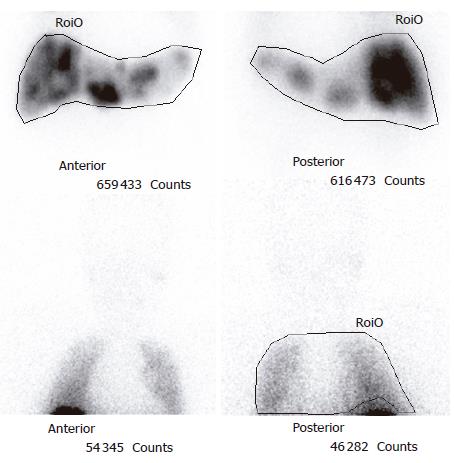
After presentation at a multidisciplinary tumor board it was decided to proceed with a trial of hepatic intra-arterial radiotherapy with yttrium-90 microspheres (SIR-Spheres). Pre-procedure evaluation with hepatic arteriography was notable for the absence of collateral vessels to the allograft, given that the liver was supplied by the donor vessels and the native gastroduodenal artery was ligated at the time of transplantation. Injection of macroaggregated albumin to determine the degree of hepato-pulmonary shunting revealed a radioactive shunt fraction of 7.3% (Figure 2). CT volumetry of the affected liver allowed calculation of a targeted tumor volume of 120.6 cm3. He underwent yttrium-90 intra-hepatic arterial injection 32 mo after liver transplantation. The patient received a dose of 1.5 GBq of isotope. The procedure was uncomplicated and he was discharged to home six hours after the procedure was completed. The patient experienced only minimal symptoms related to the treatment, including mild right upper quadrant abdominal pain and intermittent nausea. Follow-up MRI of the liver was done two months post-treatment. Pre- and post treatment MRI images (Figure 1A and B) demonstrated loss of uniform arterial enhancement and development of peripheral enhancement consistent with tumor necrosis and treatment response. The number of lesions remained stable. Post-procedure AFP levels decreased to 20 μg/L at 2 mo following the procedure. Liver function, both clinical and biochemical, was unaffected by the treatment.
Following liver transplantation, recurrence of HCC that is isolated to the liver occurs with a frequency of around 3%-5% with the current liver transplant candidate selection criteria[2-4]. Most recurrences occur within the first two years of transplantation and the two-year mortality after recurrence is high, approaching 75%[4]. Recurrent HCC may be heralded by impairment in graft function and elevation of AFP levels. Treatment measures usually include reduction of immunosuppression and local tumor therapy. Hepatic recurrence is multifocal in 60% of cases and tumor grade is T3 or greater in up to 90% of cases[3]. As a result, impaired hepatic function along with multifocal disease often limits the potential for surgical resection. Local ablative techniques such as RFA, cryoablation or percutaneous ethanol ablation require repeated administrations and also have limited efficacy when there is extensive or diffuse tumor burden. TACE has evolved as a useful tool for treatment of HCC recurrence because it can be utilized for treatment of bilateral, multifocal lesions with efficacy and limited repeated administration[6]. However, treatment requires infusion of chemotherapy and embolization of the selected hepatic artery. Systemic and local toxicity as a result of chemotherapy and hepatic ischemia is not rare[6].
This is the first known report of hepatic intra-arterial brachytherapy with yttrium 90 microsphere (SIR-Spheres) for the treatment of HCC in a liver transplant recipient. Intrahepatic yttrium 90 microsphere (SIR-Spheres) has been used for the treatment of unresectable HCC and metastatic hepatic tumors[7,8]. Yttrium-90 isotopes incorporated into a resin sphere slightly larger than the size of the capillary in the liver arterial system are delivered intra-arterially. Yttrium 90 emits beta-radiation and the permanently implanted spheres deliver a therapeutic dose radiation with an effective mean range of about 2.5 mm. The half-life of the isotope is 64 h with 95% of the dose given in the first 11 d. Higher doses of radiation are able to be delivered selectively than with external beam radiation therapy (EBRT). In addition, large segments of liver are not rendered ischemic because embolization is not a feature of the therapy. Therefore, there is less toxicity to functioning parenchyma than seen with EBRT or TACE.
Therapy is typically administered in one cycle, with a range of one-to-three cycles. Suitable candidates for treatment include patients with unilobar or bilobar disease and preserved liver function with bilirubin < 34.2 μmol/L[7]. Contrast enhanced CT imaging of the liver serves to determine tumor volume and allow calculation of radiation dose requirement. In order to minimize treatment-related toxicity, hepatic arteriography is necessary to determine arterial anatomy and administer 99 mTc-macro-aggregated albumin to calculate the shunt fraction delivered to the lungs. In most patients, arteries that arise from the hepatic arteries and supply the stomach or intestines must be embolized to prevent radiation necrosis. If the hepatic arterial supply cannot be isolated, then the patient is generally considered not to be a candidate for therapy. Because of the isolated arterial supply of the liver allograft in our patient, this was not an issue. Pulmonary shunt fraction greater than 15% on 99 mTc-macro-aggregated albumin scan predisposes to radiation pneumonitis and precludes safe treatment with yttrium 90.
The median radiation dose for a treatment is 134 Gy which is delivered in a range of 50-150 Gy[7]. The dose of yttrium 90 equates to 5-10 GBq or 2-4 million microspheres. Treatment is typically provided on an outpatient basis and does not require patient isolation because yttrium 90 is a beta emitter. Local response to treatment is demonstrated by decrease in size and tumor vascularity. Thirty-eight percent of patients experience partial response demonstrated by a decrease in tumor size[7]. In 65% of patients, response is demonstrated by substantial decrease in tumor vascularity[7]. Monitoring of tumor markers such as AFP may also be of benefit in determining response to therapy[8]. Impact on survival varies according to the severity of underlying hepatic disease but the median survival time after yttrium 90 microsphere (Theraspheres, MDS Nordion, Ottowa, Canada) treatment was 23 mo in one study of 65 patients with unresectable HCC[7]. This represents an increase in survival of 2.6-4.7 times that of historic control. One multicenter review of treated patients reported that death resulting from therapy-induced liver failure is responsible for half of the treatment-related deaths and occurs in 5% of all patients[9]. All of these liver failure deaths attributed to the yttrium 90 therapy are occurred in patients felt to be at high risk for the procedure. When compared to TACE, quality of life measures is higher at 12 mo[10]. Toxicities of yttrium-90 include worsening ascites, lower extremity edema, nausea, vomiting, fever, abdominal pain, hyperbilirubinemia and lymphopenia[7,8]. Mild adverse effects include nausea, vomiting fatigue, abdominal pain, fever[7,8]. Serious adverse effects include tumor lysis syndrome, transient elevations in liver enzymes, gastritis, gastric or duodenal perforation, cholecystitis, and radiation induced pneumonitis[7,8].
A recent multicenter review of low risk patients treated with yttrium 90 microspheres reported that liver toxicities including elevation of bilirubin and serum transaminase levels and ascites occur after treatment in 42% of patients, fifty percent of these adverse events are thought to be related to treatment, and toxicities are resolved in 78% of patients on short term follow-up[11]. Development of toxicity is associated with pretreatment bilirubin > 17.2 μmol/L and radiation dose > 137 Gy. There are no occurrences of radiation-induced liver disease or death related to treatment[11]. Another multicenter review on the use of intrahepatic yttrium 90 microspheres has reported a three-month post-treatment mortality of 18%. Ten percent of deaths are thought to be related to treatment and occur in association with at least one or more of the following risk factors: presence of infiltrative tumor, tumor bulk > 50%, albumin < 30 g/L, bilirubin ≥ 34.2 μmol/L, AST or ALT > five times the upper limit of normal, and a lung radiation dose of > 30 Gy[9]. The results of these and other reports suggest that treatment in carefully selected patients is safe and efficacious.
Hepatic intra-arterial yttrium-90 microsphere treatment was safely and effectively used in this patient with recurrent multifocal HCC after liver transplantation. Clearly, further prospective investigation is needed to determine whether this therapy can be applied with similar safety and efficacy to other patients with recurrent HCC after liver transplantation. The availability of this treatment modality in the post-transplant setting may help to prolong patient and graft survival in those recipients unfortunate enough to develop recurrent HCC who are not amenable to other treatment options.
S- Editor Pan BR L- Editor Wang XL E- Editor Bi L
| 1. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [PubMed] [DOI] [Full Text] |
| 2. | Island ER, Pomposelli J, Pomfret EA, Gordon FD, Lewis WD, Jenkins RL. Twenty-year experience with liver transplantation for hepatocellular carcinoma. Arch Surg. 2005;140:353-358. [PubMed] [DOI] [Full Text] |
| 3. | Vivarelli M, Bellusci R, Cucchetti A, Cavrini G, De Ruvo N, Aden AA, La Barba G, Brillanti S, Cavallari A. Low recurrence rate of hepatocellular carcinoma after liver transplantation: better patient selection or lower immunosuppression. Transplantation. 2002;74:1746-1751. [PubMed] [DOI] [Full Text] |
| 4. | Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, Krieger NR, Schwartz ME. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534-540. [PubMed] [DOI] [Full Text] |
| 5. | Kulik LM, Mulcahy MF, Hunter RD, Nemcek AA Jr, Abecassis MM, Salem R. Use of yttrium-90 microspheres (TheraSphere) in a patient with unresectable hepatocellular carcinoma leading to liver transplantation: a case report. Liver Transpl. 2005;11:1127-1131. [PubMed] [DOI] [Full Text] |
| 6. | Chan AO, Yuen MF, Hui CK, Tso WK, Lai CL. A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer. 2002;94:1747-1752. [PubMed] [DOI] [Full Text] |
| 7. | Carr BI. Hepatic arterial 90Yttrium glass microspheres (Therasphere) for unresectable hepatocellular carcinoma: interim safety and survival data on 65 patients. Liver Transpl. 2004;10:S107-S110. [PubMed] [DOI] [Full Text] |
| 8. | Salem R, Thurston KG, Carr BI, Goin JE, Geschwind JF. Yttrium-90 microspheres: radiation therapy for unresectable liver cancer. J Vasc Interv Radiol. 2002;13:S223-S229. [PubMed] [DOI] [Full Text] |
| 9. | Goin JE, Salem R, Carr BI, Dancey JE, Soulen MC, Geschwind JF, Goin K, Van Buskirk M, Thurston K. Treatment of unresectable hepatocellular carcinoma with intrahepatic yttrium 90 microspheres: a risk-stratification analysis. J Vasc Interv Radiol. 2005;16:195-203. [PubMed] [DOI] [Full Text] |
| 10. | Steel J, Baum A, Carr B. Quality of life in patients diagnosed with primary hepatocellular carcinoma: hepatic arterial infusion of Cisplatin versus 90-Yttrium microspheres (Therasphere). Psychooncology. 2004;13:73-79. [PubMed] [DOI] [Full Text] |
| 11. | Goin JE, Salem R, Carr BI, Dancey JE, Soulen MC, Geschwind JF, Goin K, Van Buskirk M, Thurston K. Treatment of unresectable hepatocellular carcinoma with intrahepatic yttrium 90 microspheres: factors associated with liver toxicities. J Vasc Interv Radiol. 2005;16:205-213. [PubMed] [DOI] [Full Text] |