Published online Sep 21, 2006. doi: 10.3748/wjg.v12.i35.5658
Revised: January 15, 2006
Accepted: January 24, 2006
Published online: September 21, 2006
AIM: To examine the relationship between H pylori and gastro-oesophageal reflux disease (GORD) in Iran.
METHODS: In this study 51 GORD patients (referred to endoscopy at Taleghani hospital) were compared with 49 age-sex matched controls. Diagnosis of H pylori was made by gastric mucosal biopsy and rapid urease test (positive if the result of one or both diagnostic methods was positive). Updated Sydney system was used to report histopathological changes.
RESULTS: The frequency of H pylori infection based on rapid urease test and histology was 88.2% (45) in patients and 77.6% (38) in controls, which showed no significant difference. The frequency of H pylori infection was significantly higher in the antrum than in the corpus and cardia. The mean activity, inflammation, and gastritis scores were also higher in the antrum of patients than in the antrum of controls. The mean scores were significantly higher in the corpus of controls than in the corpus of patients. Diffuse active gastritis was observed in a significantly larger number of controls, while the frequency of diffuse chronic gastritis was higher in patients. There was no significant difference in the frequency of other histological findings between patients and controls.
CONCLUSION: H pylori infection cannot prevent GORD in this region.
-
Citation: Masjedizadeh R, Hajiani E, MoezArdalan K, Samie S, Ehsani-Ardakani MJ, Daneshmand A, Zali MR.
H pylori infection and reflux oesophagitis: A case-control study. World J Gastroenterol 2006; 12(35): 5658-5662 - URL: https://www.wjgnet.com/1007-9327/full/v12/i35/5658.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i35.5658
Heartburn is a common symptom in the general population[1,2] and is associated with the development of adenocarcinoma of the oesophagus and cardia[3]. Gastritis- associated hypochlorhydria may protect against gastro-oesophageal reflux disease (GORD)[4,5]. It has hypothesized that the declined H pylori infection results in a decline in peptic ulcer and a concomitant increase in reflux disease and associated oesophageal adenocarcinoma[6,7]. However, the relationship between H pylori infection and GORD has not been established[8-11]. It was reported that prospective, large studies are needed to explore the H pylori-gastro-oesophageal disease relationship further and to avoid confusing potential benefits with known risks[9-12].
The main of this study was to investigate whether there is a difference between the frequencies of H pylori infection in cases and controls, and the possible relationship between H pylori infection and GORD.
In this study, patients with a history of heartburn, at least two times a week for a period of more than 3 mo, referred for gastrointestinal endoscopy at Taleghani Hospital, Shaheed Beheshti University of Medical Sciences, between March 2001 and February 2002, were enrolled. The reflux oesophagitis group included 51 patients (31 men and 20 women, mean age, 54.1 ± 17.2 years, range 17-80 years) with endoscopically diagnosed erosive reflux oesophagitis. Patients with a history of upper gastrointestinal (GI) surgery, malignancy, oesophageal varices, and antibiotics or bismuth consumption during the last 6 mo, together with those using H2 blockers, proton pump inhibitors (PPIs), alcohol, or non steroidal anti inflammatory drugs (NSAIDs) during the last 4 wk, were excluded from the study. The control group comprised: 49 asymptomatic patients (29 men and 20 women, mean age, 52.2 ± 17.1 years, range 18-80 years) without reflux oesophagitis, any symptom of upper GI diseases, and any lesion in their endoscopy, which was performed for other reasons (work up for iron deficiency, possible malignancy, and ERCP, sphincterotomy, or stone extraction candidates). Two cases of control group were missed during the study. The cases and controls were sex and age matched with a maximum difference of less than 3 years. Written informed consent was obtained for all upper endoscopy and biopsy procedures. This study was approved by the Ethics Committee of the Research Center of Gastroenterology and Liver Diseases, Shaheed Beheshti University of Medical Sciences.
Endoscopy was performed for both case and control groups, by two endoscopists blinded to the status of the controls and patients. The presence and grading of reflux oesophagitis were determined according to L.A. classification, from A (least severe) to D (most severe)[13]. During endoscopy, two biopsies were taken from the antrum, corpus, and cardia, and stained with standard haematoxylin/eosin and Geimsa to identify H pylori and histopathological changes. Rapid urease test was performed on the biopsy specimens from antrum, corpus, and cardia. The urease test was considered positive when the urea solution changed from yellow to pink at room temperature within 24 h. The diagnosis of H pylori infection was made by positive findings on either histology or urease test. Patients were considered to be H pylori-positive if the result of one or both diagnostic methods was positive and H pylori-negative if both methods revealed negative results.
For histopathological analysis, biopsy specimens were fixed in 40 g/L neutral-buffered formaldehyde and embedded in paraffin. Five-micron thick sections were cut from each paraffin block and stained with haematoxylin and eosin for routine histology. Two pathologists blinded to the clinical information of subjects assessed the histopathological changes independently. Updated Sydney system was used to report histopathological changes[14]. The degrees of inflammation and activity were scored from 0 (absent) to 3 (most severe). The inflammation score and activity score were summed and expressed as the gastritis score. The predominance for the anatomic regions in gastritis was determined based on the degree of inflammation in the different anatomic parts of the stomach. If the degree of inflammation was higher, the anatomic place with a higher grade of inflammation was stated as the predominant region. For the diagnosis of multifocal atrophic gastritis, we determined intestinal metaplasia or significant mucosal atrophy. Because of the low reproducibility for routine grading of mucosal atrophy, atrophy score was not used as a marker for grading[14].
Results were expressed as mean ± SD. Odd’s ratios (95% CI) were calculated to evaluate the differences in the frequency of H pylori infection and other histological findings between patient and control groups. Cochran’s Q test was used to compare the frequency of H pylori infection in the cardia, corpus, and antrum. Mann-Whitney U test was used to analyze the differences in activity, inflammation, and gastritis scores between the two groups and Friedman test was used to analyze the different scores in the cardia, corpus, and antrum. P < 0.05 was considered statistically significant.
A sample size of about 50 for patient and control groups was considered to have an 80% detecting rate (at the two-sided 5% level) with at least a 25% difference in the prevalence of H pylori between the two groups.
Among the 51 reflux oesophagitis patients, 17 (33.3%) were in grade A, 24 (47.1%) in grade B, 9 (17.6%) in grade C, and 1 (1.9%) in grade D (Table 1). Hiatal hernia was observed in 30 (58.8%) patients. The prevalence of H pylori infection is shown in Table 1. The frequency of H pylori in the antrum was significantly higher than that in the corpus and cardia of the patients (P < 0.01), while the differences were not significant in different regions of stomach of the controls, which might be due to the inadequate sample size. We were not able to find any significant difference in the frequency of H pylori infection between the two groups (OR: 2.2, 95% CI: 0.7-7.4) as shown in Table 2.
| Group | H pylori | Rut | DiagnosisH pylori | Total | ||
| Cardia | Corpus | Antrum | ||||
| Case | 27 (52.9) | 25 (49.0) | 37 (72.5) | 23 (45.1) | 45 (88.2) | 51 (100.0) |
| L.A. classification | ||||||
| A | 11 (21.6) | 8 (15.7) | 12 (23.5) | 9 (17.7) | 16 (31.4) | 17 (33.3) |
| B | 11 (21.6) | 11 (21.6) | 17 (33.4) | 12 (23.5) | 21 (41.2) | 24 (47.1) |
| C | 4 (7.8) | 5 (9.8) | 7 (13.7) | 2 (3.9) | 7 (13.7) | 9 (17.7) |
| D | 1 (1.9) | 1 (1.9) | 1 (1.9) | 0 | 1 (1.9) | 1 (1.9) |
| Control | 28 (57.1) | 27 (55.1) | 31 (63.3) | 22 (44.9) | 38 (77.6) | 49 (100.0) |
| Total | 55 (55.0) | 52 (52.0) | 68 (68.0) | 45 (45.0) | 83 (83.0) | 100 (100.0) |
| Histological finding | Case | Control | Total | OR (95% CI) |
| H pylori infection1 | 45 (88.2) | 38 (77.6) | 83 (83.0) | 2.2 (0.7-7.4) |
| Chronic inflammation in cardia | 14 (27.5) | 18 (36.7) | 32 (32.0) | 0.7 (0.3-1.7) |
| Chronic inflammation in corpus | 8 (15.7) | 22 (44.9) | 30 (30.0) | 0.2 (0.1-0.6) |
| Chronic inflammation in antrum | 28 (54.9) | 30 (61.2) | 58 (58.0) | 0.8 (0.3-1.8) |
| Overall gastritis categorization (Sydney classification) | ||||
| Diffuse chronic active gastritis | 15 (29.4) | 26 (53.1) | 41 (42.0) | 0.4 (0.2-0.9) |
| Cardia predominant chronic active gastritis | 1 (2.0) | 1 (2.0) | 2 (2.0) | 0.9 (0.0-36.3) |
| Corpus predominant chronic active gastritis | 0 | 1 (2.0) | 1 (1.0) | 0.0 (0.0-16.9) |
| Antrum predominant chronic active gastritis | 3 (5.9) | 5 (10.2) | 8 (8.0) | 0.6 (0.1-2.9) |
| Multifocal metaplastic or atrophic gastritis | 1 (2.0) | 1 (2.0) | 2 (2.0) | 0.9 (0.0-36.3) |
| Chronic carditis | 1 (2.0) | 2 (4.1) | 3 (3.0) | 0.5 (0.0-6.9) |
| Diffuse chronic gastritis | 10 (19.6) | 2 (4.1) | 12 (12.0) | 5.7 (1.1-40.4) |
| Normal | 20 (39.2) | 11 (22.4) | 31 (31.0) | 2.2 (0.9-5.8) |
| Total | 51 (100.0) | 49 (100.0) | 100 (100.0) | - |
The different histological findings in patients and controls are shown in Table 2. The frequency of chronic inflammation in the corpus was significantly higher in controls than in patients (OR: 0.2, 95% CI: 0.1-0.6). Diffuse active gastritis was also observed in controls (OR: 0.4, 95% CI: 0.2-0.9), while diffuse chronic gastritis was observed in patients (OR: 5.7, 95% CI: 1.1-40.4). The frequency of intestinal metaplasia and mucosal atrophy was not significantly different between the two groups (Table 2).
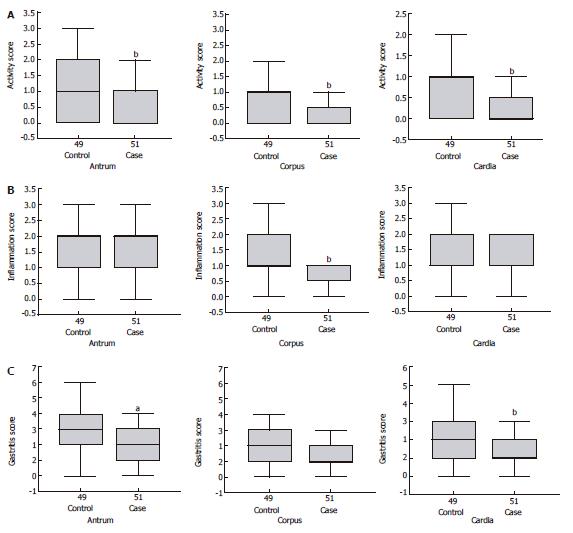
The inflammation, activity, and gastritis scores in both groups are depicted in Table 3 and Figure 1. The mean activity score in the cardia, corpus, and antrum of controls was significantly higher than that of patients (P < 0.01 or P < 0.001, Figure 1A). The inflammation score was higher in the corpus of controls than that in patients (P < 0.01, Figure 1B), while the inflammation score of the cardia and antrum was not significantly different between the two groups. Similarly gastritis score was significantly higher in controls than in patients (P < 0.01 and P < 0.05, Figure 1C).
| Group | Cardia | Corpus | Antrum | P |
| Median (range) | ||||
| Case | ||||
| H pylori n (%) | 27 (52.9) | 25 (49.0) | 37 (72.5) | 0.0051 |
| Activity score | 0 (0-2) | 0 (0-2) | 0 (0-3) | 0.0572 |
| Inflammation score | 1 (0-2) | 1 (0-2) | 2 (0-3) | 0.0002 |
| Gastritis score | 0 (0-4) | 1 (0-4) | 2 (0-6) | 0.0002 |
| Control | ||||
| H pylori n (%) | 28 (57.1) | 27 (55.1) | 31 (63.3) | 0.4651 |
| Activity score | 1 (0-2) | 1 (0-3) | 1 (0-3) | 0.0002 |
| Inflammation score | 1 (0-3) | 1 (0-3) | 2 (0-3) | 0.0002 |
| Gastritis score | 2 (0-5) | 2 (0-6) | 3 (0-6) | 0.0002 |
The mean activity score was significantly higher in the antrum than in the corpus and cardia of controls (P < 0.001, Table 3), while the differences were not significant in patients probably due to the inadequate sample size. The mean inflammation and gastritis scores were also significantly higher in antrum than in corpus and cardia of both patients and controls (P < 0.001, Table 3). These findings together with the higher frequency of H pylori infection in the antrum indicated that H pylori in antrum could induce inflammation.
Increasing attention has been paid to the relationship between H pylori infection and reflux oesophagitis in recent years. GORD is a common condition affecting 25%-40% of the population[2]. The presence of hiatal hernia[15], transient relaxation of the lower oesophageal sphincter[16,17], and impaired clearance of regurgitated gastric contents in the oesophagus[18] are considered possible causative factors for GORD.
There is evidence that infection with H pylori is the principal cause of peptic ulcer disease[10]. However, there is uncertainty about the role of this organism in GORD and the available data do not demonstrate an evident association between these two factors, although an etiologic link has been found between H pylori infection and GORD or peptic oesophagitis[10]. The prevalence of H pylori infection in patients with GORD in our study (88.2%) was higher than that reported in other studies[9], suggesting that H pylori infection is more frequent in developing countries than in industrialized countries[19].
No difference was found in the prevalence of H pylori between patients with reflux oesophagitis and controls in this study. Conflicting evidence about the association of H pylori infection with GORD has been reported and geographical location is an important determinant[9]. The pathogenic role of H pylori in reflux oesophagitis is suspected in earlier studies[20] while other studies have found no relationship between H pylori prevalence in GORD patients with that reported in other patients[10,21-34]. In contrast, the possible protective role of H pylori in reflux oesophagitis and other GORD-related diseases such as Barrett’s oesophagus and oesophageal adenocarcinoma has recently been suggested[35-39]. H pylori can cause chronic gastritis in virtually all infected people. This persistent inflammation ultimately leads to loss of the normal architecture of gastric mucosa, disappearance of gastric glands and specialized cells[40]. Diffuse active gastritis was observed in controls while diffuse chronic gastritis was observed in patients in the present study, suggesting that active inflammation might play a protective role in GORD.
Chronic antrum-predominant gastritis has been shown to be associated with secretion of acid and formation of duodenal ulcer[8,41]. In patients otherwise predisposed to reflux disease, antrum-predominant gastritis may therefore increase acid production and reflux disease development[8]. On the other hand, atrophy induced by chronic H pylori infection (chronic corpus gastritis) with decreased gastric acid production can protect against reflux oesophagitis[8,11,42]. As a consequence, the antrum predominant inflammation might be considered a factor for H pylori infection.
In conclusion, H pylori infection is not associated with DORD. Multicentre prospective studies with a larger sample size are needed to explore the relationship between H pylori infection and DORD.
We thank the National Research Department of Foodborne Diseases and the Research Center of Gastroenterology and Liver Diseases for performing this study.
S- Editor Wang GP L- Editor Wang XL E- Editor Bi L
| 1. | Locke GR 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ 3rd. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1488] [Cited by in RCA: 1380] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 2. | Jones R. Gastro-oesophageal reflux disease in general practice. Scand J Gastroenterol Suppl. 1995;211:35-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2024] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 4. | Graham DY, Yamaoka Y. H. pylori and cagA: relationships with gastric cancer, duodenal ulcer, and reflux esophagitis and its complications. Helicobacter. 1998;3:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 124] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Richter JE, Falk GW, Vaezi MF. Helicobacter pylori and gastroesophageal reflux disease: the bug may not be all bad. Am J Gastroenterol. 1998;93:1800-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | el-Serag HB, Sonnenberg A. Opposing time trends of peptic ulcer and reflux disease. Gut. 1998;43:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 257] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 7. | Pera M, Cameron AJ, Trastek VF, Carpenter HA, Zinsmeister AR. Increasing incidence of adenocarcinoma of the esophagus and esophagogastric junction. Gastroenterology. 1993;104:510-513. [PubMed] |
| 8. | Gisbert JP, Pajares JM, Losa C. Helicobacter pylori and gastroesophageal reflux disease: friends or foes. Hepatogastroenterology. 1999;46:1023-1029. [PubMed] |
| 9. | Raghunath A, Hungin AP, Wooff D, Childs S. Prevalence of Helicobacter pylori in patients with gastro-oesophageal reflux disease: systematic review. BMJ. 2003;326:737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 233] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 10. | Gisbert JP, de Pedro A, Losa C, Barreiro A, Pajares JM. Helicobacter pylori and gastroesophageal reflux disease: lack of influence of infection on twenty-four-hour esophageal pH monitoring and endoscopic findings. J Clin Gastroenterol. 2001;32:210-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Vakil NB. Review article: gastro-oesophageal reflux disease and Helicobacter pylori infection. Aliment Pharmacol Ther. 2002;16 Suppl 1:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Malfertheiner P, O'Connor HJ, Genta RM, Unge P, Axon AT. Symposium: Helicobacter pylori and clinical risks--focus on gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2002;16 Suppl 3:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Armstrong D, Bennett JR, Blum AL, Dent J, De Dombal FT, Galmiche JP, Lundell L, Margulies M, Richter JE, Spechler SJ. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996;111:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 777] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 14. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3546] [Article Influence: 122.3] [Reference Citation Analysis (3)] |
| 15. | ALLISON PR. Reflux esophagitis, sliding hiatal hernia, and the anatomy of repair. Surg Gynecol Obstet. 1951;92:419-431. [PubMed] |
| 16. | Dodds WJ, Dent J, Hogan WJ, Helm JF, Hauser R, Patel GK, Egide MS. Mechanisms of gastroesophageal reflux in patients with reflux esophagitis. N Engl J Med. 1982;307:1547-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 648] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 17. | Dent J, Holloway RH, Toouli J, Dodds WJ. Mechanisms of lower oesophageal sphincter incompetence in patients with symptomatic gastrooesophageal reflux. Gut. 1988;29:1020-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 421] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 18. | Stanciu C, Bennett JR. Oesophageal acid clearing: one factor in the production of reflux oesophagitis. Gut. 1974;15:852-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 136] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Feldman RA, Eccersley AJ, Hardie JM. Epidemiology of Helicobacter pylori: acquisition, transmission, population prevalence and disease-to-infection ratio. Br Med Bull. 1998;54:39-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Borkent MV, Beker JA. Treatment of ulcerative reflux oesophagitis with colloidal bismuth subcitrate in combination with cimetidine. Gut. 1988;29:385-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Rosioru C, Glassman MS, Halata MS, Schwarz SM. Esophagitis and Helicobacter pylori in children: incidence and therapeutic implications. Am J Gastroenterol. 1993;88:510-513. [PubMed] |
| 22. | Werdmuller BF, Loffeld RJ. Helicobacter pylori infection has no role in the pathogenesis of reflux esophagitis. Dig Dis Sci. 1997;42:103-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 125] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Walker SJ, Birch PJ, Stewart M, Stoddard CJ, Hart CA, Day DW. Patterns of colonisation of Campylobacter pylori in the oesophagus, stomach and duodenum. Gut. 1989;30:1334-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Cheng EH, Bermanski P, Silversmith M, Valenstein P, Kawanishi H. Prevalence of Campylobacter pylori in esophagitis, gastritis, and duodenal disease. Arch Intern Med. 1989;149:1373-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Befrits R, Granström M, Rylander M, Rubio C. Helicobacter pylori in 205 consecutive endoscopy patients. Scand J Infect Dis. 1993;25:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | O'Connor HJ, Cunnane K. Helicobacter pylori and gastro-oesophageal reflux disease--a prospective study. Ir J Med Sci. 1994;163:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Boixeda D, Gisbert JP, Cantón R, Alvarez Baleriola I, Gil Grande LA, Martín de Argila C. [Is there any association between Helicobacter pylori infection and peptic esophagitis]. Med Clin (Barc). 1995;105:774-777. [PubMed] |
| 28. | Liston R, Pitt MA, Banerjee AK. Reflux oesophagitis and Helicobacter pylori infection in elderly patients. Postgrad Med J. 1996;72:221-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Newton M, Bryan R, Burnham WR, Kamm MA. Evaluation of Helicobacter pylori in reflux oesophagitis and Barrett's oesophagus. Gut. 1997;40:9-13. [PubMed] |
| 30. | Csendes A, Smok G, Cerda G, Burdiles P, Mazza D, Csendes P. Prevalence of Helicobacter pylori infection in 190 control subjects and in 236 patients with gastroesophageal reflux, erosive esophagitis or Barrett's esophagus. Dis Esophagus. 1997;10:38-42. [PubMed] |
| 31. | Hackelsberger A, Schultze V, Günther T, von Arnim U, Manes G, Malfertheiner P. The prevalence of Helicobacter pylori gastritis in patients with reflux oesophagitis: a case-control study. Eur J Gastroenterol Hepatol. 1998;10:465-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Ho KY, Kang JY. Reflux esophagitis patients in Singapore have motor and acid exposure abnormalities similar to patients in the Western hemisphere. Am J Gastroenterol. 1999;94:1186-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Pieramico O, Zanetti MV. Relationship between intestinal metaplasia of the gastro-oesophageal junction, Helicobacter pylori infection and gastro-oesophageal reflux disease: a prospective study. Dig Liver Dis. 2000;32:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Oberg S, Peters JH, Nigro JJ, Theisen J, Hagen JA, DeMeester SR, Bremner CG, DeMeester TR. Helicobacter pylori is not associated with the manifestations of gastroesophageal reflux disease. Arch Surg. 1999;134:722-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Labenz J, Blum AL, Bayerdörffer E, Meining A, Stolte M, Börsch G. Curing Helicobacter pylori infection in patients with duodenal ulcer may provoke reflux esophagitis. Gastroenterology. 1997;112:1442-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 410] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 36. | Vicari JJ, Peek RM, Falk GW, Goldblum JR, Easley KA, Schnell J, Perez-Perez GI, Halter SA, Rice TW, Blaser MJ. The seroprevalence of cagA-positive Helicobacter pylori strains in the spectrum of gastroesophageal reflux disease. Gastroenterology. 1998;115:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 232] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 37. | Xia HH, Talley NJ. Helicobacter pylori infection, reflux esophagitis, and atrophic gastritis: an unexplored triangle. Am J Gastroenterol. 1998;93:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Chow WH, Blaser MJ, Blot WJ, Gammon MD, Vaughan TL, Risch HA, Perez-Perez GI, Schoenberg JB, Stanford JL, Rotterdam H. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric cardia adenocarcinoma. Cancer Res. 1998;58:588-590. [PubMed] |
| 39. | Yamaji Y, Mitsushima T, Ikuma H, Okamoto M, Yoshida H, Kawabe T, Shiratori Y, Saito K, Yokouchi K, Omata M. Inverse background of Helicobacter pylori antibody and pepsinogen in reflux oesophagitis compared with gastric cancer: analysis of 5732 Japanese subjects. Gut. 2001;49:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Kuipers EJ, Lundell L, Klinkenberg-Knol EC, Havu N, Festen HP, Liedman B, Lamers CB, Jansen JB, Dalenback J, Snel P. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med. 1996;334:1018-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 493] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 41. | Labenz J, Malfertheiner P. Helicobacter pylori in gastro-oesophageal reflux disease: causal agent, independent or protective factor. Gut. 1997;41:277-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Koike T, Ohara S, Sekine H, Iijima K, Abe Y, Kato K, Toyota T, Shimosegawa T. Helicobacter pylori infection prevents erosive reflux oesophagitis by decreasing gastric acid secretion. Gut. 2001;49:330-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 140] [Article Influence: 5.8] [Reference Citation Analysis (0)] |