INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer and the fourth most frequent cause of cancer-related deaths worldwide. Long-term survival of colorectal cancer is related to the stage of disease. Once distal metastases develop the prognosis is poor[1]. At least 40% of patients with colorectal cancer develop distal metastases and most of them die thereof[2]. Hence, innovative approaches are urgently needed to improve the treatment of advanced colorectal cancer.
There is evidence that insulin-like growth factor 1 receptor (IGF-1R) may be a promising protein for specific and targeted therapeutic approaches[3]. Several reports indicate that IGF-1R is overexpressed in the majority (> 90%) of colorectal carcinomas, most likely contributing to the aggressive growth characteristics of these tumors and the poor prognosis[4,5]. IGF-1R is a tetrameric transmembrane receptor tyrosine kinase (TK) composed of two α and two β subunits. The extracellular α subunit is responsible for ligand binding, whereas the β subunit consists of a transmembrane domain and a cytoplasmic tyrosine kinase domain[6,7]. The receptor is predominantly activated by IGF-I and II but can also be activated by insulin at a much lower affinity (500-1000 fold less). Ligand binding activates intrinsic tyrosine kinase activity, resulting in trans-β subunit autophosphorylation and stimulation of signaling cascades that include IRS-1/PI-3K/PKB/S6K and Grb2/Sos/Ras/MAPK pathways[8,9].
In general, both IGFs and IGF-1R are involved in the development and progression of several cancers[8-10]. Activation of IGF-1R by its ligands results in proliferation, survival, transformation, metastasis, and angiogenesis. Hence, abnormal or enhanced expression of IGFs and IGF-1R has been correlated with disease stage, reduced survival, metastasis development and de-differentiation of a broad variety of tumors. Obesity and diabetes are associated with an increased risk of colorectal cancer[11]. This effect seems to be due to alterations in the metabolism of endogenous hormones, including sex steroids, insulin and also activation of the IGF/IGF-receptor system which further supports the idea of the IGF/IGF-receptor system to be a promising target for colorectal cancer treatment and chemoprevention. Several studies have demonstrated the therapeutic potential of interfering with IGF-1R-mediated signaling in cancer cells in vitro and in vivo. These approaches include the use of antagonistic IGF-1R antibodies, IGF-1R antisense oligonucleotides, or IGF-1R siRNA[12-14]. Specific inhibition of IGF-1R TK activity appears to be another promising principle.
Recently, NVP-AEW541, an orally available low-mole-cular-weight pyrolo [2, 3-d] pyrimidine derivative, has been introduced as a potent and reversible inhibitor of IGF-1R tyrosine kinase activity[15]. NVP-AEW541 has been shown to be highly selective for IGF-1R-TK, as compared to both the closely related insulin receptor (InsR) and other tyrosine or serine/threonine kinases. Antitumor activity of NVP-AEW541 has already been demonstrated in fibrosarcomas and breast cancer[16]. IGFR-TK inhibition has not been evaluated for the treatment of colorectal cancer. Hence, in the present study we examined the antineoplastic potency of NVP-AEW541 both in human colorectal carcinoma cell lines and in primary cell cultures of human CRC, which shows that NVP-AEW541 potently inhibits colorectal cancer growth by inducing apoptosis and cell cycle arrest in human colorectal carcinoma cells.
MATERIALS AND METHODS
Cell lines and drugs
The human colorectal adenocarcinoma cell line HT29 was cultured in RPMI 1640 medium supplemented with 100 g/L fetal bovine serum, penicillin (100 kU/L), and streptomycin (100 mg/L). The human colorectal adenocarcinoma cell line HCT116 was grown in Dulbecco’s minimal essential medium containing 100 g/L fetal bovine serum, penicillin (100 kU/L), and streptomycin (100 mg/L). Cells were kept in a humidified atmosphere (50 mL/L CO2) at 37°C. Cells were incubated with culture medium containing NVP-AEW541 (Novartis, Basel, Switzerland). For combination treatment, cells were incubated simultaneously with NVP-AEW541 and one of the following drugs: 5-fluorouracil (Sigma, Deisenhofen, Germany), SN-38 (Rhone-Poulenc Rorer, Antony, France), cetuximab (Merck KgaA, Darmstadt, Germany) or fluvastatin (Calbiochem-Novabiochem, Bad Soden, Germany). Stock solutions (in DMSO, stored at -20°C) were diluted to the final concentration in fresh media before each experiment. In all experiments, the final DMSO concentration did not exceed 1 mL/L.
Isolation and establishment of primary cell cultures from human colorectal cancers
Cells of primary colorectal carcinomas from 8 patients (5 males, 3 females, age range 74 ± 14 years) were isolated from endoscopically taken biopsies as previously described[17]. The human tumor material was used according to the standards set by the Ethical Committee of the Charité-Universitätsmedizin Berlin, Campus Benjamin Franklin, Berlin, Germany. Cell preparation was performed by incubation (30 min/RT) with a solution containing 0.5 g/L trypsin, 0.2 g/L EDTA and 1 g/L collagenase. The isolated human colorectal carcinoma cells were maintained in Earle’s 199 medium supplemented with 200 g/L FBS, 2 mmol/L L-glutamine, 20 g/L Biotect protective medium, penicillin (100 kU/L), streptomycin (100 mg/L), 10 g/L amphotericin B, and incubated at 37°C in a humidified atmosphere (50 mL/L CO2).
Analysis of growth factor receptor expression
For analysis of growth factor receptor protein expression, cells were immunostained as previously described[18,19]. In brief, samples were fixed, permeabilized, and subsequently incubated with a polyclonal anti-EGFR or IGF-1R antibody (5 mg/L, Santa Cruz Biotechnology, Palo Alto, CA), or isotypic control rabbit IgG1 (DAKO, Hamburg, Germany). Cells were then incubated with a secondary FITC-labeled goat-anti rabbit IgG antibody (5 mg/L, BD Pharmingen, Heidelberg, Germany). Fluorescence was detected by flow cytometry on a FACSCalibur (Becton Dickinson, Heidelberg, Germany) and analyzed using Cell-Quest software.
Determination of cell number
Cell number was evaluated by crystal violet staining as previously described[20]. In brief, cells in 96-well plates were fixed with 10 mL/L glutaraldehyde and then stained with 1 g/L crystal violet. The unbound dye was removed by washing with water. Bound crystal violet was solubilized with 2 g/L Triton X-100. Light extinction increasing linearly with the cell number was analyzed at 570 nm using an ELISA reader.
Determination of cytotoxicity
Cells were incubated with 0-10 μmol/L NVP-AEW541 for 1, 6, 12 and 24 h. Release of the cytoplasmic enzyme lactate dehydrogenase (LDH), indicating cytotoxicity, was measured using a colorimetric kit from Roche (Roche Diagnostics, Mannheim, Germany) as described previously[21,22]. Background release from untreated cells was subtracted. Maximum release was measured after adding 2 g/L Triton X-100 to untreated cells. For determinations, LDH assay reagent was added to sample supernatants and incubated for 30 min at room temperature in dark. Absorbance was measured at 490 nm (reference wavelengh 690 nm).
Detection of apoptosis
Preparation of cell lysates and determination of caspase-3 activity were performed as previously described[23]. The activity of caspase-3 was calculated from the cleavage of the fluorogenic substrate DEVD-AMC (Calbiochem-Novabiochem, Bad Soden, Germany). The proportion of apoptotic cells was determined by quantifying the percentage of sub-G1 (hypodiploid) cells after flow cytometric analysis of isolated propidium iodide-stained nuclei[24].
Cell cycle analysis
Cell cycle analysis was performed by the method of Vindelov and Christensen[25]. Cells were trypsinized, washed, and the nuclei were isolated using CycleTest PLUS DNA Reagent Kit (Becton Dickinson). DNA was stained with propidium iodide according to the manufacturer’s instructions. DNA content of the nuclei was detected by flow cytometry and analyzed using CellFit software (Becton Dickinson).
Western blotting
Western blotting was performed as previously described[23]. Blots were blocked in 50 g/L non-fat dry milk for 1 h, and then incubated at 4°C overnight with anti-phospho-Akt, Akt (both 1:1000, Cell Signaling, Beverly, MA), Bax (1:1000, Santa Cruz Biotechnology, CA), Bcl-2 (1:200, Novo Castra Laboratories, Newcastle upon Tyne, UK), COX-2 (1:200), cyclin D1 (1:100), p21Waf1/CIP1 (1:200), IGF-1R (1:200), phospho-ERK1/2, ERK1/2 (1:1000, Santa Cruz Biotechnology, CA), or p27Kip1 (1:2500, Becton Dickinson). β-actin (1:5000, Sigma) served as a loading control.
Statistical analysis
If not stated otherwise, means of four independent experiments ± SE were shown. Individual drug treatment was compared by the unpaired, two-tailed Mann-Whitney U-test. P < 0.05 was considered statistically significant.
RESULTS
Expression of IGF-1R in colorectal carcinoma cells
Expression of IGF-1R and epidermal growth factor receptor (EGFR) was investigated in human colorectal carcinoma cells. Protein expression of IGF-1R was detected in both cell lines. In addition, expression of EGFR protein was detected in both cell lines (Figure 1A). IGF-1R protein expression of NVP-AEW541-treated colorectal cancer cells was determined by Western blotting. HT-29 cells incubated with NVP-AEW541 (0-10 μmol/L) for 48 h did not abolish the expression of IGF-1R. By contrast even after treatment with 10 μmol/L of NVP-AEW541, a robust expression of IGF-1R protein could still be observed (Figure 1B). IGF-1R and EGFR expression was confirmed in the investigated 8 primary colorectal cancer cultures by RT-PCR using established primers (not shown)[18,22].
Figure 1 Flow cytometric analysis of IGF-1R and EGFR protein expression in HT-29 (A) and HCT-116 (B) cells.
Cells were stained with antibodies against either IGF-1R (black areas) or EGFR (grey areas). Black lines: isotypic controls.
NVP-AEW541-induced growth inhibition of colorectal carcinoma cells
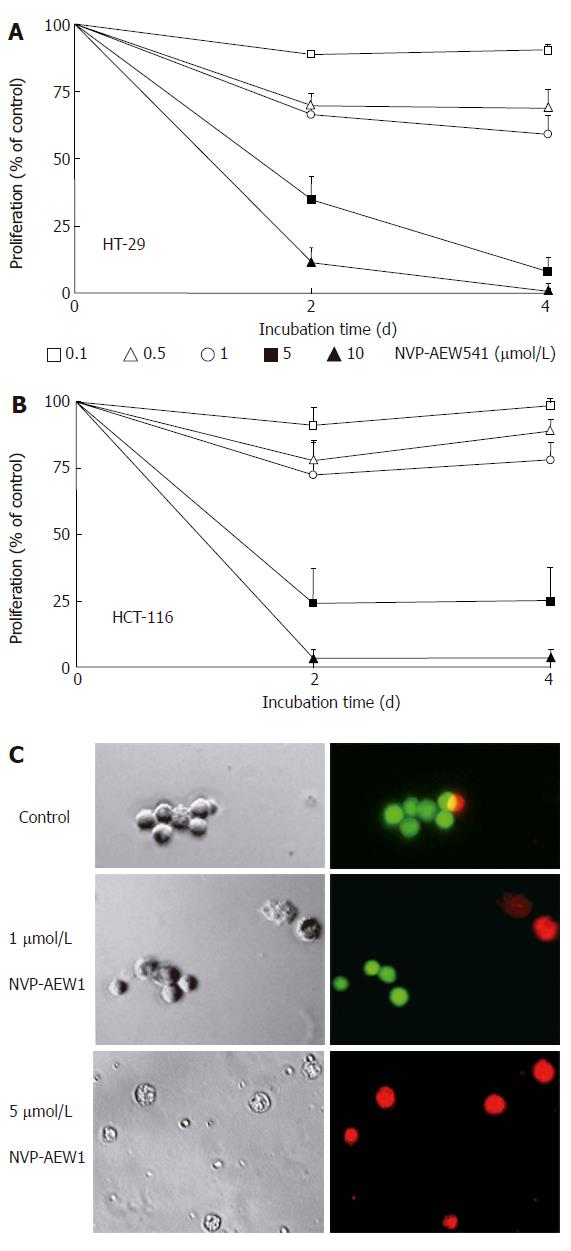
Cell number changes caused by IGF-1R-TK inhibition with NVP-AEW541 were studied by crystal violet assays. NVP-AEW541 time- and dose-dependently inhibited the growth of HT-29 and HCT-116 cells (Figure 2A and B). The IC50 values of NVP-AEW541 were 1.7 ± 0.4 μmol/L (HT-29) and 2.5 ± 0.4 μmol/L (HCT-116), as determined after 4 d of incubation.
Figure 2 Effects of NVP-AEW541 on HT-29 (A) and HCT-116 (B) cell growth as well as induction of cell death and morphological changes of isolated primary colorectal cancer cells (C).
After 4 d of incubation with rising concentrations of NVP-AEW541, the number of HT-29 (A) and HCT-116 (B) cells decreased by > 95%, as determined by crystal violet staining (mean ± SE, n = 4). In both cell lines statistical significance (P < 0.05) of growth inhibition by NVP-AEW541 was shown for concentrations of 0.5-10 μmol/L. After 3 d of incubation with 0-5 μmol/L NVP-AEW541, the induction of cell death and morphological changes of isolated primary colorectal cancer cells was determined by Live/Dead-fluorescence microscopy (C). Viable cells are stained green, while cells with impaired cell membrane appear red. Phase-contrast images and corresponding fluorescence micrographs of a representative preparation (out of 6 NVP-AEW541-sensitive primary cell cultures) are depicted.
In line with our findings in permanent cell lines, NVP-AEW541 treatment (0-5 μmol/L) reduced the cell number of primary cultures of human colorectal carcinomas in a dose-dependent manner. After 3 d of incubation a cell number reduction of 47.3% ± 2.4% was detected by direct cell counting in six NVP-AEW541-sensitive primary culture preparations. Two out of the investigated 8 primary cultures displayed only a weak growth inhibition of 12% ± 4%. In treatment-sensitive primary cultures NVP-AEW541 also altered the morphology of the remaining cells, which appeared shrunken and flat. Propidium iodide-positive staining of primary culture cells revealed that NVP-AEW541 treatment led to a loss of cell membrane integrity indicating cell death or that these cells were in the process of dying, respectively (Figure 2C).
Antineoplastic potency of NVP-AEW541 in combination with cytostatics, cetuximab or the HMG-CoA reductase inhibitor fluvastatin
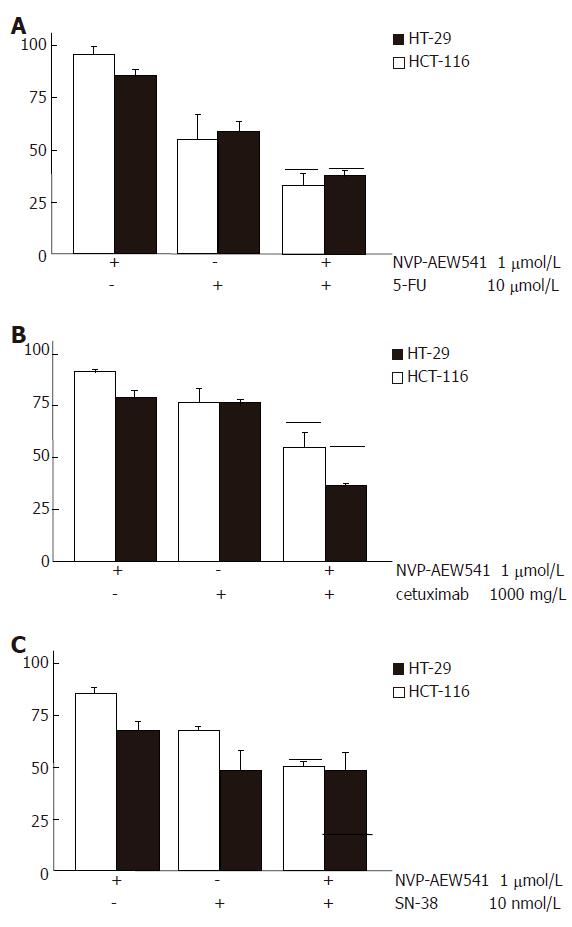
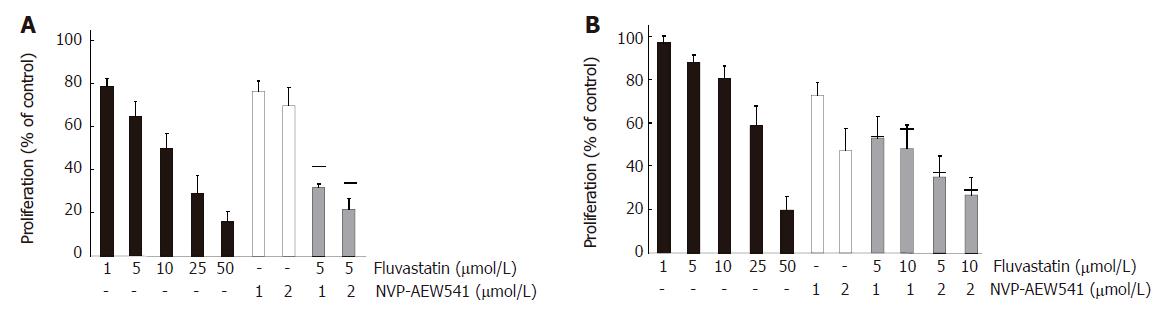
To test whether combination treatment of NVP-AEW541 with either 5-fluorouracil (5-FU), SN-38, or whether the humanized monoclonal anti-EGFR antibody cetuximab may lead to additive antiproliferative effects, HT29 and HCT-116 cells were co-treated for 96 h with sub-IC50 concentrations of NVP-AEW541 (1 μmol/L) plus either 5-FU (10 μmol/L), or cetuximab (1000 mg/L), or SN-38 (10 nmol/L). In both colorectal carcinoma cell models combination treatment with NVP-AEW541 plus either 5-FU (Figure 3A) or cetuximab (Figure 3B) resulted in additive or even over-additive growth inhibitory effects. When NVP-AEW541 was combined with SN-38, additive antiproliferative effects were only observed in HCT-116 cells (Figure 3C). We additionally investigated the anti-proliferative potency of the HMG-CoAR inhibitor fluva-statin, either alone or in combination with NVP-AEW541 in colorectal carcinoma cells (Figure 4). Fluvastatin (0-50 μmol/L) caused a dose-dependent growth inhibition of more than 80% both in HCT-116 cells and in HT-29 cells. Combinations of sub-IC50 concentrations of fluvastatin and NVP-AEW541 resulted in an additive growth inhibition of either colorectal cancer model.
Figure 3 Augmented growth inhibition of combination treatment with NVP-AEW541 plus either 5-FU (A), or SN-38 (B) or cetuximab (C) (mean ± SE, n = 4-6).
A: Combination treatment with sub-IC50 concentrations of NVP-AEW541 plus 5-FU led to synergistic growth inhibition of colorectal cancer cells; B: Combination treatment with sub-IC50 concentrations of NVP-AEW541 plus the humanized EGFR-antibody cetuximab resulted in slightly over-additive antiprolifertive effects; C: Co-treatment with sub-IC50 concentrations of NVP-AEW541 and SN-38 resulted in additive growth inhibition of HCT-116 cells, while no additive growth inhibition was detected in HT-29 cells. Black bars indicate the values of the calculated additive growth inhibition. Data are given as percentage of untreated controls.
Figure 4 Additive growth inhibition by NVP-AEW541 plus fluvastatin (mean ± SE, n = 3-5).
Fluvastatin (1-50 μmol/L) induced a dose-dependent growth inhibition of HCT-116 (A) and HT-29 (B) cells by > 80% when applied for 3 d. Moreover, combination treatment with sub-IC50 concentrations of fluvastatin and NVP-AEW541 led to (over-)additive growth inhibition after 3 d of treatment. Black bars indicate the values of the calculated additive growth inhibition.
NVP-AEW541-induced apoptosis in colorectal cancer cells
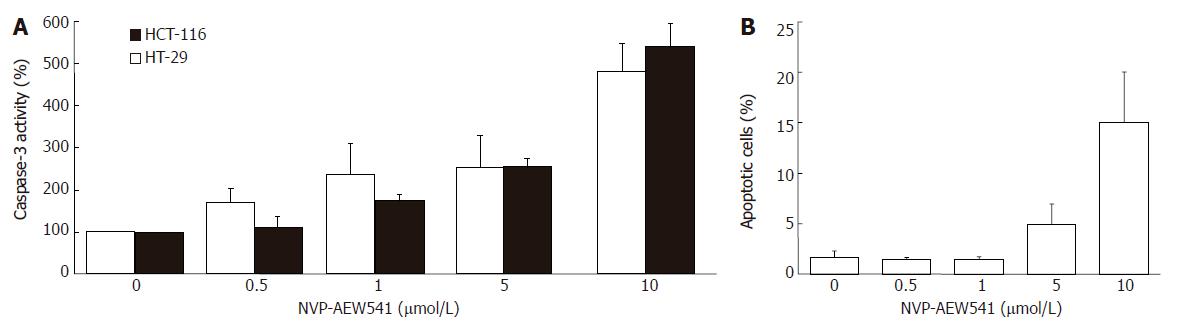
NVP-AEW541 dose-dependently induced a significant increase of caspase-3 activity after 24 h of incubation (Figure 5A). Compared to control cells, an increase of up to 500% was observed. The dose-dependent induction of apoptosis by NVP-AEW541 became also apparent by flow cytometrically monitoring nuclear degradation after 72 h of treatment (Figure 5B).
Figure 5 Apoptosis induction by NVP-AEW541 (mean ± SE, n = 4).
A: After HT-29 and HCT-116 cells were incubated with 0.5-10 μmol/L NVP-AEW541 for 24 h, a dose-dependent induction in caspase-3 activation was observed; B: After 72 h of NVP-AEW541 treatment an increased proportion of apoptotic cells measured as subdiploidy was observed in HT-29 cells. aP < 0.05 vs untreated controls.
NVP-AEW541-induced cell cycle arrest in colorectal carcinoma cells.
To test whether an induction of cell cycle arrest contributed to the antiproliferative potency of NVP-AEW541 in colorectal carcinoma cells, we performed cell cycle analyses. NVP-AEW541 dose-dependently arrested HT-29 and HCT-116 cells in the G1/G0 phase of the cell cycle after 24 h of treatment, thereby decreasing the proportion of cells in the S and G2/M phases (Figure 6).
Figure 6 Effects of NVP-AEW541 on the cell cycle of colorectal carcinoma cells (means ± SE, n = 4).
After 24 h incubation with NVP-AEW541, a dose-dependent accumulation of HT-29 (A) and HCT-116 (B) cells was observed in the G0/G1 phase of the cell cycle (white bars). Proportion of cells in the S and G2/M phase (grey and black bars) decreased. aP < 0.05 vs untreated controls.
Cytoxicity of NVP-AEW541
Cytotoxicity of NVP-AEW541 was determined by measuring LDH release. HT29 and HCT-116 cells incubated with 1-10 μmol/L NVP-AEW541 for 1, 6 or 12 h did not result in a measurable increase in LDH release, indicating that NVP-AEW541 did not directly affect cell membrane integrity. After 24 h of incubation a slight but not significant increase in LDH-release of about 3% was observed at 10 μmol/L NVP-AEW541, indicating that even at high concentrations NVP-AEW541 did not cause immediate necrotic/cytotoxic effects in colorectal cancer cells (data not shown).
NVP-AEW541-induced modulation of cell cycle and apoptosis-related signaling molecules
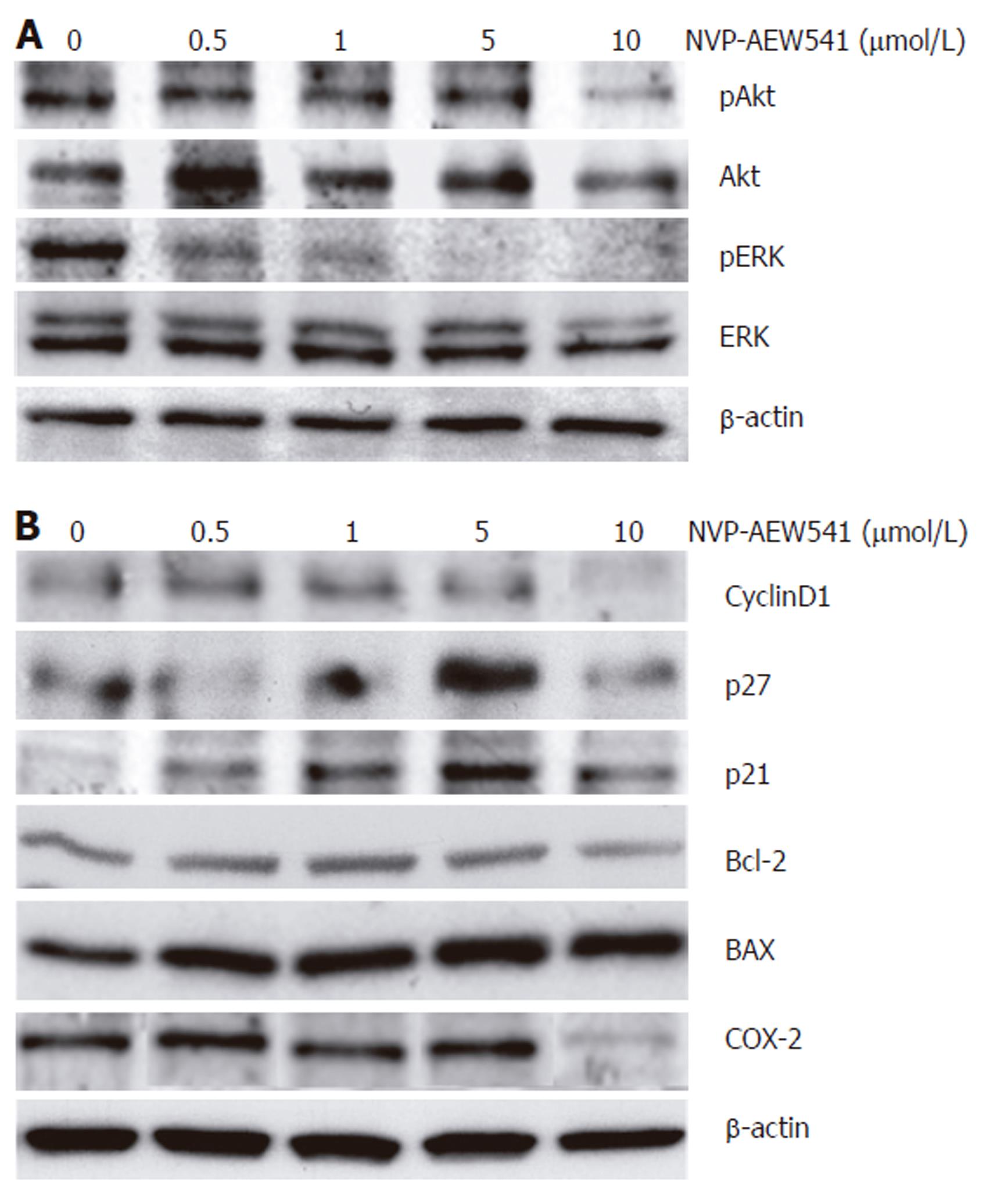
The effects of NVP-AEW541 on the phosphorylation of both ERK1/2 and its upstream regulator Akt/PKB were investigated to elucidate the signaling pathways modulated by IGF-1R-TK inhibition. NVP-AEW541 treatment dose-dependently decreased the phosphorylation of both mitogenic and antiapoptotic ERK1/2 MAPK as well as Akt/PKB (Figure 7A). Next, the expression of cell cycle-related proteins was investigated to explore the pathway downstream of NVP-AEW541-induced dephosphorylation of Akt/PKB and ERK1/2, known to be causative for cell cycle arrest. NVP-AEW541 decreased the expression of cyclin D1 but increased the expression of the CDK inhibitors p21Waf1/CIP1 and p27Kip1. These data suggest that NVP-AEW541-induced cell cycle arrest was mediated by p21Waf1/CIP1 and p27Kip1 induction, resulting in a decrease of cyclin D1.
Figure 7 Effects of NVP-AEW541 on the expression and phosphorylation of apoptosis- and cell cycle- related proteins.
Modulation of protein phosphorylation or protein expression by NVP-AEW541 treatment was analyzed by Western blotting. A: NVP-AEW541 treatment (24 h) induced a dose-dependent dephosphorylation of mitogenic ERK1/2; B: NVP-AEW541 dose-dependently increased the expression of the proapoptotic Bax protein, while the expression of the antiapoptotic protein Bcl-2 slightly decreased. The cell cycle promoter cyclin D1 was down-regulated by NVP-AEW541 treatment, while the cell cycle inhibitors p21Waf1/CIP1 and p27Kip1 were up-regulated. Moreover, COX-2 expression was down-regulated by NVP-AEW541. Expression of β-actin was used as a loading control.
To survey the proapoptotic signaling pathways modulated by IGF-1R-TK inhibition with NVP-AEW541 in CRC cells, we also investigated the effects of NVP-AEW541 on the expression pattern of Bcl-2 and Bax. Treatment with NVP-AEW541 dose-dependently increased the expression of the proapoptotic Bax protein, while the expression of the antiapoptotic protein Bcl-2 slightly decreased. Finally, it was proved that NVP-AEW541 could downregulate the expression of cyclooxygenase 2 (COX-2) in colorectal cancer cells (Figure 7B).
DISCUSSION
Treatment options for advanced colorectal cancer (CRC) are unsatisfactory. Thus, there is a strong need for effective novel treatment strategies of CRC. A novel approach for CRC treatment may be the interruption of IGF/IGF-receptor signaling system, which is known to have strong stimulatory effects on cancer cell growth. The protective and mitogenic effects of the IGF/IGF-receptor system involve the constitutive activation of antiapoptotic proteins as well as cell cycle promoting signaling. A tight association between IGF-receptor signaling and regulation of cell growth and apoptosis in CRCs which commonly overexpress IGF-receptors has been described[4,5,26].
Most fast growing cancers produce and release growth factors and thereby auto-stimulate their growth. This also holds true for CRC in which epidermal growth factor (EGF), transforming growth factor α (TGF-α), insulin-like growth factor (IGF) and vascular endothelial growth factor (VEGF) are produced and secreted to promote CRC growth[27]. Therefore, the antiproliferative effects of the novel IGF-1R tyrosine kinase inhibitor NVP-AEW541 were investigated under serum-containing conditions (e.g. in the presence of growth factors like EGF, IGF and TGF-α). Our study demonstrated that inhibition of IGF-1R tyrosine kinase activity by NVP-AEW541 might be suitable for novel targeted therapy of CRC. By abrogating the protective and mitogenic effects of IGF-R signaling, CRC cell growth was potently inhibited by NVP-AEW541. The antineoplastic effects of NVP-AEW541 were based on a pronounced induction of cell cycle arrest and apoptosis. Accompanying or dose-limiting cytotoxicity was not observed, underlining the specific mode of action of the drug. Our findings are in line with recent observations on IGF-R inhibition in other cancer models. Although applying other approaches for IGF-R inhibition, these studies have also shown the strong antineoplastic potency of the interruption of IGF-R signaling without dose-limiting toxicity in several other cancer models[16,28,29].
Re-initialisation of apoptosis and induction of cell cycle arrest are valuable features for a successful anti-cancer agent. On the one hand, apoptotic cell death is not accompanied with undesired immunological reactions, which occur upon treatment with unspecific and cytotoxic agents. On the other hand, apoptosis-sensitization and cell cycle arresting effects offer possibilities for powerful combination treatments. In this respect, we investigated combination treatments of NVP-AEW541 and clinically relevant cytostatics with different modes of action. The tested combinations of DNA strand-breaking pyrimidine analog 5-fluorouracil (5-FU) and topoisomerase-I inhibitor SN-38, except for the combination of NVP-AEW541 and SN-38 in HT29 cells, resulted in additive antiproliferative effects. The additive antineoplastic potency appeared to be independent of the mode of action of the respective cytostatic drug. Our data support the notion that NVP-AEW541 is a promising new agent for potentiation of antitumor efficay of the established cytostatic CRC treatment.
It has been pointed out that the antiapoptotic potency of IGF/IGFR-signaling might interfere with strategies that target other tyrosine kinases such as EGFR-TK. Hence, the antineoplastic potency of EGFR blockade may well be underestimated when examined under conditions where IGF-1R is fully functional. IGF-1R is capable of transactivating EGFR-TK and abrogating the antiproliferative effects of EGFR-antibody treatment[30-32]. As the CRC cell models used in this study were shown to express both IGF-1R and EGFR, we studied the effects of targeting both growth factor receptors by combination treatment. Treating CRC cells with sub-IC50 concentrations of both NVP-AEW541 and humanized anti-EGFR-antibody cetuximab enhanced the antiproliferative effect as compared to the effect of either agent alone. Thus, NVP-AEW541 qualifies as a promising substance for combination treatment strategies to overcome the compensatory effects of mitogenic crosstalks between IGF-1R and EGFR in CRC.
Drug resistance is one of the major problems of chemotherapy. Potential mechanisms of drug resistance include the activation of Ras/Raf/Mek/ERK signal transduction cascade and the increase of cholesterol levels in cancer cells, both being controlled by isoprenoids[33]. The production of isoprenoids is catalyzed by 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoAR) which may therefore be a rational molecular target for innovative antineoplastic treatment of colorectal cancer. Fluvastatin is an effective inhibitor of HMG-CoAR and has already been shown to inhibit tumor cell growth[34]. In the present study, we have demonstrated the antineoplastic effect of fluvastatin alone and in combination with NVP-AEW541 in colorectal cancer cells and the antiproliferative effect of NVP-AEW541 augmented by fluvastatin, suggesting that combining NVP-AEW541 and fluvastatin may be a promising approach for dual targeting treatment strategies in colorectal cancer disease.
The mechanisms underlying the antiproliferative action of NVP-AEW541 in colorectal cancer cells were further characterized. NVP-AEW541 induced cell cycle arrest in the G1/G0-phase in both CRC cell lines, suggesting that the drug acts at the G1/S checkpoint. A G1/S cell cycle arrest induced by inhibition of IGFR signaling has been described in other fast growing cancers[35,36]. Moreover, we observed a definite rise in apoptotic cells after treatment with NVP-AEW541. While the induction of apoptosis is a well-known effect occurring upon inhibition of IGFR-signaling, the underlying mechanisms have been poorly characterized[37-39]. Our present results suggest that activation of caspase-3 is involved in NVP-AEW541-induced apoptosis of CRC cells. The proapoptotic protein BAX was upregulated during NVP-AEW541-induced apoptosis, while Bcl-2, the protective and antiapoptotic counterpart of Bax, was downregulated. Our findings suggest that involvement of mitochondrial pathways leads to NVP-AEW541-mediated apoptosis.
As activated IGF-1R induces the Ras-Raf-MEK-ERK signaling pathway with subsequent induction of cyclin D1 expression, we analyzed NVP-AEW541-induced changes of Akt/PKB, ERK1/2 activity and p21Waf1/CIP1, p27Kip1 as well as cyclin D1 expression as previously described[40]. In agreement with previous observations in non-colorectal tumor models[16], we found that NVP-AEW541 could dephosphorylate IGF-1R as well as ERK1/2 MAPK and AKT/PKB of colorectal cancer cells.
IGF-IR has at least three survival signals that are able to protect cancer cells from apoptosis, namely PI-33K/AKT and MAPK/ERK signaling pathways, and a third one that results in the mitochondrial translocation of Raf 1, the so called 14-3-3 pathway[41]. Simultaneous inactivation of two of these pathways is required to inhibit IGF-1R capacity of protecting cells from apoptotic injuries[42]. By showing the simultaneous inactivation of AKT/PKB and ERK1/2 MAPK by NVP-AEW541, we hypothezise that blockade of these two survival pathways is directly involved in the successful inhibition of colorectal cancer cell growth by NVP-AEW541.
Defective function of cell cycle regulators is a main cause for tumor development and progression. For example, the cell cycle promoter cyclin D1 is frequently overexpressed in CRC. Successful therapeutic strategies have to balance or bypass the impaired signaling. In the present study, NVP-AEW541 treatment raised the expression of the cell cycle-inhibiting molecules p21Waf1/CIP1 and p27Kip1, while it decreased the expression of cyclin D1. Finally, the expression of COX-2 which is known to be upregulated during colorectal carcinogenesis and plays an important role in colorectal cancer growth, was suppressed by IGFR-TK inhibition as previously described[43]. Thus, NVP-AEW541-induced COX-2 suppression may well contribute to its antineoplastic potency in colorectal cancer. Since both COX-2 and IGFR are up-regulated during colorectal carcinogenesis, NVP-AEW541 appears to be a promising chemopreventive agent in patients at risk for CRC.
Primary cell cultures of human colorectal cancers were established as a tool to design a rational individual medical treatment of an individual patient. The primary goal was to study NVP-AEW541’s antineoplastic potency in a bench to bedside approach, as permanent cell lines may represent well-suited but nevertheless non-representative models of colorectal cancers. Moreover, chemosensitivity testing of primary cultures was performed to establish a new method for predicting the response of an individual patient to a certain drug. Attempts to predict individual responses have already been undertaken for breast cancer and colorectal cancer, respectively[44,45]. Such an approach may pave the way to an individualized medical treatment of cancer patients. The importance of predictive testing is further supported by our finding that two out of the tested eight primary cell cultures showed only a weak response to NVP-AEW541 treatment. Nevertheless, NVP-AEW541 is a promising compound for future colorectal cancer treatment, as 75% of the investigated primary colorectal cancers could be effectively treated with the drug.
In conclusion, the IGFR-TK inhibitor NVP-AEW541 potently inhibits the growth of human colorectal cancer cells by inducing both cell cycle arrest and apoptosis without eliciting unspecific cytotoxicity. Furthermore, the compound is well-suited for combination treatment approaches. Thus, inhibition of IGFR-signaling by NVP-AEW541 is a promising targeted anticancer strategy for colorectal carcinoma and should be tested in future clinical trials. Moreover, investigations should be pursued to modulate the IGF/IGFR system as a possible means of chemoprevention of colorectal cancer in patients at risk.