Published online Sep 14, 2006. doi: 10.3748/wjg.v12.i34.5523
Revised: May 28, 2006
Accepted: June 14, 2006
Published online: September 14, 2006
AIM: To investigate the effects of luminal exposure to H2O2 and two related thiol oxidizing agents on basal and stimulated chloride secretion in native colon using electrophysiological and pharmacological approaches.
METHODS: Unstripped rat distal colon segments were mounted in Ussing chambers. Potential difference, calculated resistance and short-circuit current across unstripped colon segments were monitored with a dual voltage/current clamp. Paracellular permeability was assessed by measuring the mucosa-to-serosa flux of a fluorescent probe (FITC).
RESULTS: Luminal exposure to hydrogen peroxide transitorily stimulated chloride secretion without altering barrier function. This stimulatory effect could be blocked by basolateral atropine but not indomethacin. The cysteine and methionine oxidizing compounds, phenylarsine oxide and chloramine T respectively, mimicked the effect of H2O2, except for a drop in transcolonic resistance after 30 min. In contrast to the observed stimulatory effect on basal secretion, cAMP-stimulated electrogenic ion transport was blunted by luminal H2O2. However, the Ca2+-activated response remained unchanged.
CONCLUSION: H2O2 may be an important selective modulator of intestinal ion and water secretion in certain pathologic conditions such as inflammation or ischemia-reperfusion by multiple mechanisms.
- Citation: Mayol JM, Adame-Navarrete Y, Alarma-Estrany P, Molina-Roldan E, Huete-Toral F, Fernandez-Represa JA. Luminal oxidants selectively modulate electrogenic ion transport in rat colon. World J Gastroenterol 2006; 12(34): 5523-5527
- URL: https://www.wjgnet.com/1007-9327/full/v12/i34/5523.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i34.5523
The colonic lumen is an extraordinarily aggressive milieu containing high concentrations of oxidizing compounds[1] as a result of the digestion of nutrients, the presence of dietary oxidants and the metabolic activity of resident colonic flora. Moreover, many gastrointestinal diseases, such as intestinal ischemia, ischemic colitis or inflammatory bowel disease, are associated with an increase in the concentration of both reactive oxygen and nitrogen species in the intestinal mucosa[2], generated by either activated neutrophils infiltrating the colonic crypts or epithelial cells submitted to ischemia/reperfusion. Therefore, colonic epithelial cells and submucosal tissue are under continuous luminal oxidative stress in states of health and disease, but little is known about the impact of luminal oxidants on mucosal functions in intact native mammalian colon since most studies have been carried out in cell lines.
Oxidants have been shown to interfere with epithelial ion transport and barrier function. In intestinal model cell lines, addition of hydrogen peroxide, either basolaterally or apically, first activates vectorial Cl- transport and then inhibits cAMP-activated anion secretion. The inhibitory effect on the forskolin-stimulated response appears to be the result of the blockade of apical Cl- conductance and the inhibition of Na+-K+-ATPase activity[3]. In animal models, serosal application of H2O2, at micromolar concentrations, to muscle-stripped rat colon evokes a transient increase in anion secretion[4]. Yet, the role of luminal oxidants on stimulated transport in intact native colon remains poorly understood. Therefore, our aim was to investigate the effect of luminal H2O2 on basal and stimulated electrogenic ion transport in rat distal colon.
Male Sprague-Dawley rats weighing 250-350 g were killed by intraperitoneal administration of sodium pentobarbital. Animals were handled in accordance with the protocols approved by Comisión de Investigación-Hospital Clínico San Carlos, in compliance with both Spain’s and European Union’s regulations for the use of animals in biomedical research.
The method to obtain samples for electrophysiological studies has been reported elsewhere[5,6]. In brief, a 5-cm segment of distal colon was surgically removed and placed in iced buffer solution. The specimen was opened along its mesenteric side. Unstripped colonic segments were mounted in modified chambers (Costar, San Diego CA) with a surface area of 0.64 cm2. These chambers could provide individualized access to both the luminal and the serosal compartments of the colon. Both chambers were bathed with an identical volume (5 mL) of the buffer solution containing 122.0 mmol/L NaCl, 2.0 mmol/L CaCl2, 1.3 mmol/L MgSO4, 5.0 mmol/L KCl, 20.0 mmol/L glucose and 25.0 mmol/L NaHCO3 (pH 7.4 when gassed with 950 mL/L de O2 and 50 mL/L CO2 at 37°C).
Two Ag/AgCl electrodes connected to a dual voltage-current clamp (World Precision Instruments ECV 4000, Sarasota-FL, USA) were placed in the apical and basolateral chambers. Spontaneous transepithelial potential difference (E0; lumen negative, in mV) could therefore be monitored. Potential difference values were corrected for the junction potentials (< 0.1 mV) between the luminal and the serosal solutions. Two additional electrodes were used to apply a 50 μA current through the mounted colon. The resulting potential difference was measured (E50). Transcolonic resistance (TR, in Ω·cm2) was calculated with E0 and E50 values by Ohm’s law, reflecting epithelial viability and intestinal barrier function. Short circuit current (Isc, in μA/cm2) could be obtained with E0 and TR values by Ohm´s law, indicating the amount of electrical current needed to nullify the spontaneous potential difference between the apical and the basolateral surfaces.
Electrophysiological experiments were carried out after the samples were mounted in the modified chambers and bathed in buffer solution until stable electrical activity was reached. The response of rat colon exposed to luminal oxidants to the muscarinic blocker (atropine) was studied after it was added to the basolateral chamber in the continued presence of both compounds. Similarly, the effect of two different secretagogues (forskolin and carbachol) on ion transport was assessed following the same protocol (luminal incubation + continued presence of the oxidant).
Paracellular permeability was assessed by measuring the mucosal-to-serosal flux of fluorescein isothiocyanate (FITC; MW: 376.3) as previously described[6]. Paired rat distal colon segments were mounted in chambers and bilaterally incubated in regular buffer for 10 min for equilibration. Subsequently, the apical buffer was replaced with a FITC-containing solution (140 μmol/L) with or without hydrogen peroxide. Basolateral buffer aliquots were collected at 0, 5, 15, 30 and 60 min after apical buffer replacement and the fluorescent emission at 520 nm after excitation at 480 nm was measured with a spectrofluometer (Bio-Tek FL600 Fluorescence Microplate Reader, Bio-Tek Instruments GmbH, Bad Friedrichshall, Germany). A calibration curve (fluorescence vs FITC concentration) was generated to calculate the concentration of FITC in the serosal chamber. The apparent permeability coefficient (Papp) was calculated according to the equation Papp(cm/seg) = (ct2/ct1)·V·A-1·C0-1, where (ct2/ct1) is the net increase in FITC concentration in the serosal buffer in a given interval of time (seconds), V is the volume (in milliliters) of the basolateral compartment, A is the surface of the colon segment (in square centimeters) and C0 is the concentration in the apical chamber.
Cyclic AMP and Ca2+-dependent secretion was stimulated with forskolin (10 μmol/L) and carbachol (100 μmol/L), respectively. Phenylarsine oxide (PAO, 0.2 mmol/L) and chloramine T (ClT, 5 mmol/L) were used as non-physiologic thiol oxidants. For muscarinic receptor inhibition, atropine at a concentration of 1 mg/L was employed. Indomethacin (1-10 μmol/L) was used for blockage of cyclo-oxygenase activity. Forskolin and indomethacin were added to both chambers (luminal and serosal). However, exposition to the muscarinic agonist and its antagonist was only from the serosal side. All the compounds were obtained from Sigma, Madrid-Spain.
Results were presented as mean ± SD. Student’s t and repeated measure ANOVA tests were used for statistical comparisons when indicated. P < 0.05 was considered statistically significant.
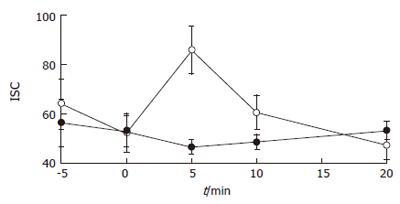
Luminal exposure to H2O2 (up to 10 mmol/L) for 30 min did not alter either transcolonic resistance (TR of H2O2-treated samples = 92.2% ± 11.7% of controls; n = 6 for each group; NS) or paracellular permeability (Papp = 1.24 × 10-6 ± 0.16 × 10-6 for control vs 0.95 × 10-6 ± 0.35 × 10-6 for 10 mmol/L H2O2; n = 6 for each group, NS). However, as shown in Figure 1, a transient increase in Isc occurred 5 min after the addition of the oxidant to the apical chamber (peak Isc = 46.7 ± 2.8 μA/cm2 for control samples, n = 5; vs 85.9 ± 9.6 μA/cm2 for 10 mmol/L H2O2; n = 6, P < 0.01). This secretory response was dose-dependent (from 100 μmol/L to 10 mmol/L) and peaked at a concentration of -8 mmol/L (ΔIsc = 32.3 ± 3.4 μA/cm2 at 8 mmol/L; n = 4 for all groups; P < 0.05 for the comparison between controls and oxidant-treated samples). However, it was not specific for H2O2 since two widely used cysteine and methionine oxidizing agents, phenylarsine and chloramine T, at concentrations used by other investigators to study epithelial barrier function in cell lines (0.2 mmol/L and 5 mmol/L respectively), generated the same secretory response after 5 min but induced a slight drop in transcolonic resistance 30 min after addition (Table 1).
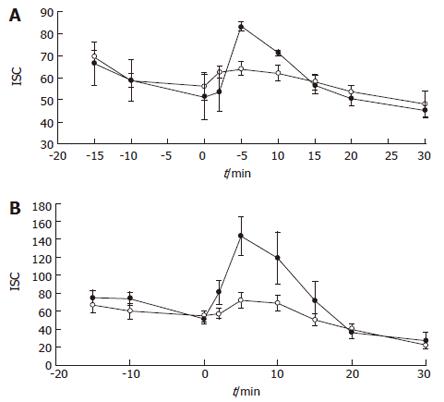
In order to investigate the implication of different secretagogues in H2O2-induced secretion, colonic samples were first incubated with varying concentrations of indomethacin (1-10 μmol/L) and subsequently exposed to the three oxidizing compounds. No inhibition of the Isc rise caused by 10 mmol/L H2O2 was observed. For example, samples incubated with 2 μmol/L indomethacin after addition of 10 mmol/L H2O2 yielded a peak Isc of 110 ± 13.7 μA/cm2, whereas for controls the peak Isc was 134 ± 14.2 μA/cm2 (n = 9 for each group; NS). The same result was observed with the other two oxidants (data not shown). Since other mediators, such as acetylcholine, might be involved in the observed effect, the response to luminal oxidants after muscarinic receptor blockade was investigated. Interestingly, the secretory response elicited by the three compounds was suppressed by basolateral preincubation with atropine (1 g/L). The ΔIsc in atropine-treated samples after exposure to 10 mmol/L H2O2 was -1.3 ± 8.6 μA/cm2, whereas in samples incubated in regular buffer the ΔIsc in response to the same concentration of H2O2 was 55.2 ± 13.7 μA/cm2 (n = 6, P < 0.01). Both PAO and chloramine T displayed a similar behaviour (Figures 2A and 2B).
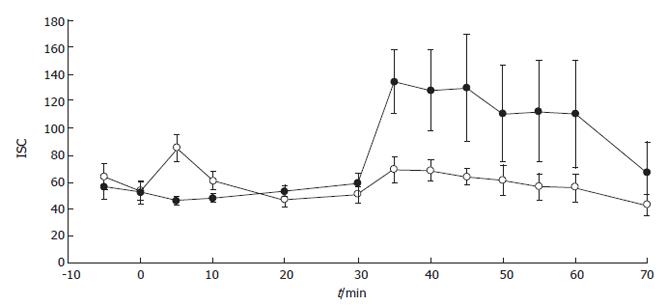
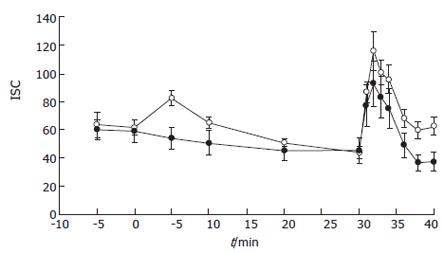
Subsequently, the effect of luminal H2O2 on stimulated secretion was studied. After 30 min of incubation with 10 mmol/L H2O2, when no drop in permeability occurred (TR after incubation for 30 min = 116.6 ± 15.8 Ω·cm2 H2O2-treated samples vs 87.8 ± 19.7 Ω·cm2 for control; n = 6 and n = 5 respectively; NS), forskolin stimulated-secretion was inhibited (Figure 3) (maximal Isc = 69.3 ± 9.4 μA/cm2 for H2O2-treated samples vs 134.1 ± 23.1 μA/cm2 for control (n = 6 and n = 5 respectively; P < 0.01). This inhibitory action was dose-dependent with an IC50-0.7 mmol/L. Since Ca2+-dependent agonists could activate distinct signaling pathways and membrane transporters to generate electrogenic ion transport, we studied the effect of 10 mmol/L H2O2 on the carbachol-stimulated secretory response. In this case, samples exposed to the oxidant luminally or to regular buffer responded to the Ca2+ agonist in the same fashion (Figure 4).
Even under normal conditions, colonic epithelial cells are continuously exposed to luminal oxidants from different sources[7,8]. Since these compounds may interfere with epithelial functions, further understanding of the specific effects of reactive oxygen species on ion transport is necessary to design therapeutic strategies to prevent cell dysfunctions, such as hypersecretion and energy depletion, during inflammation or ischemia[1,2] . In this study, we showed that luminal hydrogen peroxide, within a physiologically relevant concentration range, could transitorily activate chloride secretion without altering either electrical resistance or paracellular permeability in rat distal colon. This effect might be due to either direct stimulation of membrane transport proteins or due to the activation of intracellular second messengers in epithelial cells. In fact, mucosal and serosal addition of H2O2 could potentiate Cl- secretion in a synergistic fashion[9] in T84 cells previously stimulated with cAMP-agonists, suggesting that the Ca2+ signaling pathway might be involved in H2O2-triggered secretory response.
However, the maximal Isc induced by H2O2 in the T84 cell line is rather small compared to that observed in our model. Sugi et al[10] suggested that oxidants might enhance the response to secretagogues by priming of transport proteins in epithelial cell membrane. Interestingly, our results suggest that sudden increases in the concentration of luminal oxidants could activate secretion by releasing acetylcholine rather than by directly stimulating epithelial cells, which is independent from the generation of prostaglandins. This assertion is supported by the fact that the rise in Isc induced by three different oxidants-hydrogen peroxide, phenylarsine oxide and chloramine T, is almost completely suppressed by incubation with basolateral atropine but not with indomethacin at several concentrations. These findings are in contrast with the observations reported by Karayalcin et al[11], who found that basolateral hydrogen peroxide stimulates indomethacin-inhibitable secretion in stripped rat colon, which can be only partially blocked by atropine. Likewise, Tamai et al[4] showed that H2O2 increases Isc probably by stimulating release of arachidonate metabolites and neurotransmitters. This divergent finding may be due, at least in part, to the different “in vitro” models used. We studied the unstripped rat distal colon[12,13] because it could more accurately reflect the native tissue “in vivo” than the mucosa-submucosa preparations. Furthermore, Tamai et al[4] and Karayalcin et al[11] have used lower H2O2 concentrations and applied the oxidant to the serosal aspect of the stripped colonic wall.
An alternate explanation would imply that luminal H2O2 exerts a distinct action on each signaling pathway. That is, it might be priming the Ca2+-dependent pathway while blocking the cAMP-dependent pathway. Thus, the stimulatory action of prostaglandins on colonic epithelial cells would be abolished and therefore, would become “invisible”. To further support this hypothesis, previous studies in T84 cells have shown that incubation with H2O2 blocks chloride secretion stimulated by the cAMP agonist forskolin[3]. Similarly, we found that H2O2 could inhibit the cAMP-dependent secretory response, but not the activation of electrogenic ion transport by carbachol. In fact, the response to the Ca2+ agonist was slightly augmented. This finding points at a cyclic nucleotide-dependent membrane transport protein as the target for luminal oxidants. From previous studies, one can speculate that apical cAMP-dependent Cl- channels are the most likely sites of action for these compounds[3,14]. The Na+-K+-ATPase is another potential target for oxidazing agents[3]. However, the discrepancy between the observed inhibition of the cAMP-dependent increase in vectorial anion transport and the absence of such an action on Ca2+-stimulated secretion renders this explanation unlikely.
In conclusion, our results suggest that H2O2 may be an important selective modulator of intestinal ion and water secretion in certain pathologic conditions such as inflammation or ischemia-reperfusion by multiple mechanisms. Irrespective of the underlying mechanism of activation, the fact that an increase in the luminal concentration of reactive oxygen species triggers chloride secretion may be teleologically explained as a defensive mechanism for flushing microorganisms and toxic byproducts of bacterial metabolism away from the colonic lumen to dilute their concentration.
S- Editor Pan BR L- Editor Wang XL E- Editor Bi L
| 1. | Halliwell B, Zhao K, Whiteman M. The gastrointestinal tract: a major site of antioxidant action. Free Radic Res. 2000;33:819-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 351] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 2. | Jourd'heuil D, Morise Z, Conner EM, Grisham MB. Oxidants, transcription factors, and intestinal inflammation. J Clin Gastroenterol. 1997;25 Suppl 1:S61-S72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | DuVall MD, Guo Y, Matalon S. Hydrogen peroxide inhibits cAMP-induced Cl- secretion across colonic epithelial cells. Am J Physiol. 1998;275:C1313-C1322. [PubMed] |
| 4. | Tamai H, Kachur JF, Baron DA, Grisham MB, Gaginella TS. Monochloramine, a neutrophil-derived oxidant, stimulates rat colonic secretion. J Pharmacol Exp Ther. 1991;257:887-894. [PubMed] |
| 5. | Mayol JM, Alarma-Estrany P, O'Brien TC, Song JC, Prasad M, Adame-Navarrete Y, Fernández-Represa JA, Mun EC, Matthews JB. Electrogenic ion transport in mammalian colon involves an ammonia-sensitive apical membrane K+ conductance. Dig Dis Sci. 2003;48:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Mayol JM, Alarma-Estrany P, Adame-Navarrete Y, Roldan EM, Toral FH, Fernandez-Represa JA. Effects of luminal ATPase inhibitors on electrogenic ion transport in rat distal colon. J Surg Res. 2005;129:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Erhardt JG, Lim SS, Bode JC, Bode C. A diet rich in fat and poor in dietary fiber increases the in vitro formation of reactive oxygen species in human feces. J Nutr. 1997;127:706-709. [PubMed] |
| 8. | Nathan CF. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987;80:1550-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 607] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 9. | Nguyen TD, Canada AT. Modulation of human colonic T84 cell secretion by hydrogen peroxide. Biochem Pharmacol. 1994;47:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Sugi K, Musch MW, Di A, Nelson DJ, Chang EB. Oxidants potentiate Ca(2+)- and cAMP-stimulated Cl(-) secretion in intestinal epithelial T84 cells. Gastroenterology. 2001;120:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Karayalcin SS, Sturbaum CW, Wachsman JT, Cha JH, Powell DW. Hydrogen peroxide stimulates rat colonic prostaglandin production and alters electrolyte transport. J Clin Invest. 1990;86:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Stoner MC, Scherr AM, Lee JA, Wolfe LG, Kellum JM. Nitric oxide is a neurotransmitter in the chloride secretory response to serotonin in rat colon. Surgery. 2000;128:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Prasad M, Ito S, Silen W. Functional studies of in vitro rat distal colon before and after restitution. Surgery. 1997;121:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Jeulin C, Fournier J, Marano F, Dazy AC. Effects of hydroxyl radicals on outwardly rectifying chloride channels in a cultured human bronchial cell line (16HBE14o-). Pflugers Arch. 2000;439:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |