Published online Sep 14, 2006. doi: 10.3748/wjg.v12.i34.5473
Revised: May 28, 2006
Accepted: June 16, 2006
Published online: September 14, 2006
AIM: To investigate the relationship between the -765G > C COX-2 polymorphism and the development of different gastric lesions: atrophy or intestinal metaplasia and gastric adenocarcinoma.
METHODS: A cross-sectional study was performed involving 320 Portuguese individuals (210 without evidence of neoplastic disease, 73 patients with gastric adenocarcinomas and 37 with atrophy or intestinal metaplasia) using a PCR-RFLP method.
RESULTS: -765C allele was overrepresented in the patients with gastric adenocarcinoma (51%) when compared either with the control group (38%) or patients with atrophy or intestinal metaplasia (27%). Callele was found to be very common in our population (0.22), and a multivariate logistic regression analysis revealed nearly 3-fold increased risk for the progression to gastric adenocarcinoma in patients with atrophy or intestinal metaplasia carrying the -765C allele (OR = 2.67, 95% CI = 1.03-6.93; P = 0.04).
CONCLUSION: -765C carrier status should be considered as another susceptibility marker for gastric adenocarcinoma development in patients with atrophy or intestinal metaplasia.
- Citation: Pereira C, Sousa H, Ferreira P, Fragoso M, Moreira-Dias L, Lopes C, Medeiros R, Dinis-Ribeiro M. -765G > C COX-2 polymorphism may be a susceptibility marker for gastric adenocarcinoma in patients with atrophy or intestinal metaplasia. World J Gastroenterol 2006; 12(34): 5473-5478
- URL: https://www.wjgnet.com/1007-9327/full/v12/i34/5473.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i34.5473
Gastric adenocarcinoma mortality rates have been decreasing in Europe[1], although, in Portugal it still remains one of the leading causes of cancer-related deaths (third in men and fourth in women). Portugal has one of the highest mortality rates of Europe and the highest in southern Europe, with values of 33.2 and 20.8 per 100 000/year in men and women, respectively[2].
Cyclooxygenase (COX), also known as prostaglandin endoperoxide synthase, is a rate-limiting enzyme that converts free arachidonic acid into important prostanoids (PGs) and eicosanoids such as prostaglandin H2[3]. There are at least two isoforms of COX identified[4]: COX-1 is expressed constitutively in most cell types and is thought to be responsible for the maintenance of vascular homeostasis and gastroprotection[3,4]; and, COX-2, the inducible isoform of the enzyme, undetectable in most cells is readily induced by bacterial lipopolysaccharide (LPS), cytokine, growth factors, mitogens and tumor promoters[3-6].
Enhanced expression of COX-2 has been observed in several forms of cancer[7-14], including gastric cancer and precancerous tissues[15-18]. COX-2 over-expression plays an important role in the inhibition of apoptosis, tumor growth, angiogenesis, invasion and metastasis, which are considered to be important steps in cancer development[3,15,16,19-24].
Single Nucleotide Polymorphisms (SNP) are the most common form of genetic variants of the human genome[25], some of which might have functional effects on the susceptibility to the development of human cancers[26-33] by modifying the transcriptional activation.
Several polymorphisms in COX-2 have been identified so far. However, only a few seemed to have a functional effect on the transcription. Recently, Papafili et al[34] described a new polymorphism in the promoter region of COX-2, characterized by a guanine (G) to cytosine (C) transition at position -765 (-765G > C). This polymorphism appears to disrupt a Stimulatory protein 1 (Sp1) binding site, which is considered to be a positive activator of transcription and leads to a 30% reduction of the COX-2 promoter activity in vitro[34]. With this evidence, only few investigations have been done involving COX-2 polymorphisms either in cancer related studies[25,35-42] or other diseases[34,43-45].
The aim of this study was to determine the allelic frequencies of the -765G > C COX-2 polymorphism in a northern Portuguese population and to investigate its association with the development of gastric lesions, such as, atrophic gastritis or intestinal metaplasia and gastric adenocarcinoma.
The -765G > C COX-2 polymorphism was evaluated in a cross-sectional study performed in healthy individuals without clinical evidence of cancer (n = 210) and patients with known gastric lesions (n = 110), both from the northern region of Portugal attended at the Portuguese Institute of Oncology (Porto, Portugal).
The control group was formed by 75 females and 135 males (64%) with a median age of 51 years old. Patients were divided according to the type of lesion presented upon histopathological diagnosis after endoscopic multiple biopsies. Seventy three patients displayed lesions as severe as high-grade non-invasive neoplasia and intestinal type invasive gastric adenocarcinoma and 37 with lesions, such as atrophy or intestinal metaplasia that belong to a standardized follow-up since 2001[46]. The three different groups are characterized in Table 1. The group of patients with gastric adenocarcinoma included 27 females and 31 males (53%) with a mean age of 54 years and, and the group of patients with atrophy or intestinal metaplasia included 21 females and 13 males (38%) with a mean age of 61 years.
| Control | Gastricadenocarcinoma | Atrophy or intestinalmetaplasia | |
| n = 210 | n = 73 | n = 37 | |
| Age | |||
| (mean ± SD) | 49.5 ± 18.0 | 54.2 ± 11.3 | 60.7 ± 10.9 |
| Male Gender n (%) | 135 (64) | 36 (49) | 15 (40) |
| Atrophy n (%) | Na | Na | 3 (8) |
| Complete IM n (%) | Na | Na | 4 (11) |
| Incomplete IM n (%) | Na | Na | 30 (81) |
All samples were obtained with the permissions of the individuals before their inclusion in the study after informed consent according to the Declaration of Helsinki.
Blood samples were collected with a standard venipuncture technique using EDTA containing tubes. Genomic DNA was extracted from peripheral blood leukocytes by a standard Salting-out protocol[47].
The analysis of the -765G > C polymorphism was performed by PCR-based restriction fragment length polymorphism (PCR-RFLP) as previously described[43]. The primers used in the amplification were: CX2A (forward): 5'-ATT CTG GCC ATC GCC GCT TC-3' and CX2B (reverse) 5'-CTC CTT GTT TCT TGG AAA GAG ACG-3' (Metabion, Martinsried, Deutschland). The amplification conditions were 95°C during 10 min for the initial denaturation step, followed by 35 cycles of denaturation at 94°C (1 min), annealing at 59°C (1 min) and extension at 72°C (1 min). The final extension step consisted 10 min at 72°C. As a negative control PCR mix without DNA sample was used to ensure contamination free PCR product. Reaction products were digested with Bsh1236I restriction endonuclease (Fermentas, Vilnius, Lithuania) during 8 h at 37°C.
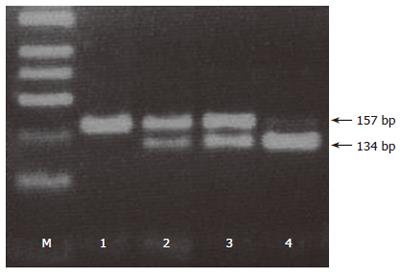
Results were observed in 3% agarose gel stained with ethidium bromide (Figure 1). Fragment sizes of 134 + 23 bp indicated a wild-type homozygous -756GG genotype, and an uncut fragment of 157 bp indicated the homozygous -765CC genotype. The presence of all the three bands (23, 134, and 157 bp) indicated a heterozygous -765GC genotype. The 23 bp fragment, resulting from the Bsh1236I restriction can not be distinguished from the primer-dimmer band in the agarose gel. Analysis of genotypes was independently performed by two of the authors (C.P. and P.F.). Cases with nonconcordant results between the two observers, or with the absence of a PCR product were rejected. Also a second PCR-RFLP analysis was performed in ten per cent of all samples to confirm the genotype.
Individual’s age and gender, type of gastric lesion (gastric adenocarcinoma or atrophy or intestinal metaplasia) or its absence, and COX-2 alleles (G, C).
Data analysis was performed using the computer software Statistical Package for Social Sciences-SPSS for Windows (version 11.5). Chi-square analysis was used to compare categorical variables, using a 5% level of significance. Multivariate logistic regression analysis was used to estimate odds ratio (OR) and its 95% confidence interval (CI) as a measure of the association between Allele C carrier and the risk for the development of gastric lesion. Gender and age were included in multivariate analysis, and assessment for interaction was considered in the model.
The distribution of -765G > C COX-2 genotypes is shown in Table 2. The frequency of the -765GG, GC and CC genotypes were 62%, 32% and 6%, respectively in controls, 49%, 44% and 7% in patients with gastric adenocarcinoma and 73%, 24% and 3% in patients with atrophic gastritis or intestinal metaplasia. All genotypic distributions are in Hardy-Weinberg equilibrium (P > 0.05). -765C carriers were more frequently found among those with gastric adenocarcinoma (P = 0.04) than the other groups.
| Genotype | Controls(n = 210) | Atrophy or intestinalmetaplasia(n = 37) | Gastricadenocarcinoma(n = 73) | ||
| n (%) | P1 | n (%) | P2 | n (%) | |
| GG | 130 (62) | 0.197 | 27 (73) | 0.018 | 36 (49) |
| GC | 67 (32) | 0.357 | 9 (24) | 0.046 | 32 (44) |
| CC | 13 (6) | 0.398 | 1 (3) | 0.339 | 5 (7) |
| C carrier | 80 (38) | 0.197 | 10 (27) | 0.018 | 37 (51) |
Table 3 describes the Odds Ratio for the development of atrophic gastritis or intestinal metaplasia and gastric adenocarcinoma. We found no statistically significant risk for the development of either atrophy or intestinal metaplasia (OR = 0.60, 95% CI = 0.28-1.31; P = 0.20) or gastric adenocarcinoma (OR = 1.67, 95% CI = 0.98-2.86; P = 0.06). Although the results for the development of gastric lesions were not statistically significant, we observed a possible protective role for -765C carriers, and when the same analysis was adjusted for age and gender by a multivariate logistic regression analysis this protective effect disappeared (OR = 0.95, 95% CI = 0.91-0.99; P = 0.01). In contrast, we observed a nearly 3-fold increased risk for the progression of atrophy or intestinal metaplasia into gastric adenocarcinoma (OR = 2.67, 95% CI = 1.03-6.93; P = 0.04).
Furthermore, when we evaluated the distribution of gender in the two groups, atrophy or intestinal metaplasia and gastric adenocarcinoma, no statistically significant differences were observed (P = 0.38).
Enhanced expression of COX-2 gene has been reported in several forms of cancer, including gastric precancerous and adenocarcinoma tissues[15-18]. This evidence suggests a role of COX-2 in the carcinogenesis pathway, such as in the inhibition of apoptosis, tumour growth, angiogenesis, invasion and metastasis[3,15,16,19-24]. A -765G > C polymorphism on the promoter region of COX-2 gene disrupts the Sp1 binding site[34] that may alter the susceptibility to develop cancer[36]. Our results revealed that C allele is extremely common (22%) in our population. Although only a few studies have been developed involving this COX-2 polymorphism, the frequencies of the polymorphic variant seems to vary, especially among different ethnic populations (Table 4). These studies revealed that the C allele is more frequent in Western countries, in Europe and America, than in Asian countries. Moreover, our results for the genotype frequencies are in concordance with other previously reported data in Caucasian populations.
In the present study, -765C carriers were slightly overrepresented in the control population (38%) when compared with patients with atrophy or intestinal metaplasia (27%). In fact, although not statistically significant, our results revealed a possible protective role for -765C carriers. Nevertheless, when adjusted for age and gender this protective role disappears (OR = 0.95, 95% CI = 0.91-0.99, P = 0.01) suggesting that this variant does not influence the development of gastric lesions such as atrophy or intestinal metaplasia. Although, the protective role is in agreement with previous studies, the small sample size may raise some statistical concerns to this observation. Papafili et al[34] showed that the promoter activity of the -765C allele is reduced to about 30% when compared to the -765G. In addition, Ulrich et al[38] revealed a marginal protection for the development of colorectal adenomatous and hyperplastic polyps when associated with the -765CC genotype. Thus, these results confirm the evidence that the depletion of the Sp1 binding site, considered a positive activator of COX-2 transcription, caused by the -765G > C transition, modifies the transcriptional activation of COX-2[34].
Gastric cancer developed upon a multistep process from chronic active gastritis, gastric glandular atrophy (GA), intestinal metaplasia (IM), dysplasia and gastric cancer[46]. In a recent work, it was suggested that COX-2 expression increases as it progresses from initial gastric lesions to gastric cancer, providing evidence that COX-2 might contribute to an early event in gastric carcinogenesis[48]. Another approach attempt to understand the influence of the COX-2 -765G > C polymorphism has in the progression from atrophy or intestinal metaplasia lesions to gastric adenocarcinoma. We observed a nearly 3-fold increased risk of progression from gastric lesions into gastric cancer (OR = 2.67, 95% CI = 1.03-6.93; P = 0.04). This result is consistent with previously cancer-related studies that also revealed that -765C allele carriers had increased risk for the development of those diseases[36,49]. More recently, Zhang et al[42] described a 2-fold increased risk for the development of esophageal squamous cell carcinoma due to increased expression of COX-2 mRNA in -765G > C heterozygous. Although the exact molecular mechanism by which COX-2 polymorphism may affect the risk of gastric adenocarcinoma development is still unclear, studies in the COX-2 promoter revealed that COX-2 transcription is activated by E2 promoter binding factor 1 (E2F1)[50], which is dependent on the transactivation and DNA-binding domains of E2F1[51]. So the ability of this polymorphism to create an E2F binding site, essential for the expression of several genes[43], might help us to understand why we observed increased risk.
In conclusion, all these findings suggest that different physiological/pathological conditions, as well as cell type, could determine the influence that -765G > C COX-2 polymorphism has in the development of human diseases, by the modification of the binding sites for the transcription factors[18]. The contribution of genetic polymorphism to the risk of gastric adenocarcinoma may be dependent on the population in study, as well on several environmental and dietary factors that influence that population. So, we hypothesize that each population has to evaluate its own genetic profile for cancer risk that may help to understand the geographic and racial differences reported for gastric adenocarcinoma[52]. Furthermore, COX-2 polymorphisms may be involved in different individual drug response[53,54] and may be explored as for in clinical trials to select those individual to be submitted to COX-2 inhibition. Moreover, the definition of a pharmacogenomic profile using molecular studies may help to the development of a personalized treatment or quimioprevention.
To the best of our knowledge, this is the first report that evaluates the -765G > C COX-2 polymorphisms and gastric adenocarcinoma development worldwide, which also considers the progression from gastric lesions, such as atrophy or intestinal metaplasia, to gastric adenocarcinoma. We theorise that once the lesions are installed, -765C carriers are at risk of progression into gastric adenocarcinoma. However, our results should be cautiously interpreted as they report a cross-sectional design. Therefore, we suggest that -765G > C polymorphism should be used in a large cohort study among patients with atrophy or intestinal metaplasia as a susceptibility marker for gastric adenocarcinoma and to confirm the real meaning of this genetic alteration in gastric adenocarcinoma development.
S- Editor Pan BR L- Editor Karam SM E- Editor Bi L
| 1. | Pinheiro PS, Tyczyński JE, Bray F, Amado J, Matos E, Parkin DM. Cancer incidence and mortality in Portugal. Eur J Cancer. 2003;39:2507-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13558] [Article Influence: 677.9] [Reference Citation Analysis (1)] |
| 3. | Wang D, Mann JR, DuBois RN. The role of prostaglandins and other eicosanoids in the gastrointestinal tract. Gastroenterology. 2005;128:1445-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Markenson J. Clinical Implications of Cyclooxygenase Enzymes: COX-1/COX-2 Role of the New NSAIDs. Cancer Control. 1999;6:22-25. [PubMed] |
| 5. | McAdam BF, Mardini IA, Habib A, Burke A, Lawson JA, Kapoor S, FitzGerald GA. Effect of regulated expression of human cyclooxygenase isoforms on eicosanoid and isoeicosanoid production in inflammation. J Clin Invest. 2000;105:1473-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 166] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Bakhle YS. COX-2 and cancer: a new approach to an old problem. Br J Pharmacol. 2001;134:1137-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, Edelstein D, Soslow RA, Koki AT, Woerner BM. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:991-994. [PubMed] |
| 8. | Gupta S, Srivastava M, Ahmad N, Bostwick DG, Mukhtar H. Over-expression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate. 2000;42:73-78. [PubMed] [DOI] [Full Text] |
| 9. | Boström PJ, Aaltonen V, Söderström KO, Uotila P, Laato M. Expression of cyclooxygenase-1 and -2 in urinary bladder carcinomas in vivo and in vitro and prostaglandin E2 synthesis in cultured bladder cancer cells. Pathology. 2001;33:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Kokawa A, Kondo H, Gotoda T, Ono H, Saito D, Nakadaira S, Kosuge T, Yoshida S. Increased expression of cyclooxygenase-2 in human pancreatic neoplasms and potential for chemoprevention by cyclooxygenase inhibitors. Cancer. 2001;91:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 11. | Ferrandina G, Legge F, Ranelletti FO, Zannoni GF, Maggiano N, Evangelisti A, Mancuso S, Scambia G, Lauriola L. Cyclooxygenase-2 expression in endometrial carcinoma: correlation with clinicopathologic parameters and clinical outcome. Cancer. 2002;95:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Niki T, Kohno T, Iba S, Moriya Y, Takahashi Y, Saito M, Maeshima A, Yamada T, Matsuno Y, Fukayama M. Frequent co-localization of Cox-2 and laminin-5 gamma2 chain at the invasive front of early-stage lung adenocarcinomas. Am J Pathol. 2002;160:1129-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Garcea G, Sharma RA, Dennison A, Steward WP, Gescher A, Berry DP. Molecular biomarkers of colorectal carcinogenesis and their role in surveillance and early intervention. Eur J Cancer. 2003;39:1041-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Brown JR, DuBois RN. Cyclooxygenase as a target in lung cancer. Clin Cancer Res. 2004;10:4266s-4269s. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Li HX, Chang XM, Song ZJ, He SX. Correlation between expression of cyclooxygenase-2 and angiogenesis in human gastric adenocarcinoma. World J Gastroenterol. 2003;9:674-677. [PubMed] |
| 16. | Chen XL, Su BS, Sun RQ, Zhang J, Wang YL. Relationship between expression and distribution of cyclooxygenase-2 and bcl-2 in human gastric adenocarcinoma. World J Gastroenterol. 2005;11:1228-1231. [PubMed] |
| 17. | Yu JR, Wu YJ, Qin Q, Lu KZ, Yan S, Liu XS, Zheng SS. Expression of cyclooxygenase-2 in gastric cancer and its relation to liver metastasis and long-term prognosis. World J Gastroenterol. 2005;11:4908-4911. [PubMed] |
| 18. | Zhang JT, Wang MW, Zhu ZL, Huo XH, Chu JK, Cui DS, Qiao L, Yu J. Increased expression of cyclooxygenase-2 in first-degree relatives of gastric cancer patients. World J Gastroenterol. 2005;11:4918-4922. [PubMed] |
| 19. | Leahy KM, Koki AT, Masferrer JL. Role of cyclooxygenases in angiogenesis. Curr Med Chem. 2000;7:1163-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Uefuji K, Ichikura T, Mochizuki H. Cyclooxygenase-2 expression is related to prostaglandin biosynthesis and angiogenesis in human gastric cancer. Clin Cancer Res. 2000;6:135-138. [PubMed] |
| 21. | Dempke W, Rie C, Grothey A, Schmoll HJ. Cyclooxygenase-2: a novel target for cancer chemotherapy. J Cancer Res Clin Oncol. 2001;127:411-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 285] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 22. | Howe LR, Subbaramaiah K, Brown AM, Dannenberg AJ. Cyclooxygenase-2: a target for the prevention and treatment of breast cancer. Endocr Relat Cancer. 2001;8:97-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 228] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Cao Y, Prescott SM. Many actions of cyclooxygenase-2 in cellular dynamics and in cancer. J Cell Physiol. 2002;190:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 339] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 24. | Wang D, DuBois RN. Cyclooxygenase 2-derived prostaglandin E2 regulates the angiogenic switch. Proc Natl Acad Sci USA. 2004;101:415-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Hu Z, Miao X, Ma H, Wang X, Tan W, Wei Q, Lin D, Shen H. A common polymorphism in the 3'UTR of cyclooxygenase 2/prostaglandin synthase 2 gene and risk of lung cancer in a Chinese population. Lung Cancer. 2005;48:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Medeiros R, Morais A, Vasconcelos A, Costa S, Pinto D, Oliveira J, Lopes C. The role of vitamin D receptor gene polymorphisms in the susceptibility to prostate cancer of a southern European population. J Hum Genet. 2002;47:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Ferreira PM, Medeiros R, Vasconcelos A, Costa S, Pinto D, Morais A, Oliveira J, Lopes C. Association between CYP2E1 polymorphisms and susceptibility to prostate cancer. Eur J Cancer Prev. 2003;12:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Ribeiro R, Vasconcelos A, Costa S, Pinto D, Morais A, Oliveira J, Lobo F, Lopes C, Medeiros R. Overexpressing leptin genetic polymorphism (-2548 G/A) is associated with susceptibility to prostate cancer and risk of advanced disease. Prostate. 2004;59:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Pinto D, Vasconcelos A, Costa S, Pereira D, Rodrigues H, Lopes C, Medeiros R. HER2 polymorphism and breast cancer risk in Portugal. Eur J Cancer Prev. 2004;13:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Freitas-Silva M, Pereira D, Coelho C, Bicho M, Lopes C, Medeiros R. Angiotensin I-converting enzyme gene insertion/deletion polymorphism and endometrial human cancer in normotensive and hypertensive women. Cancer Genet Cytogenet. 2004;155:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Coelho A, Matos A, Catarino R, Pinto D, Pereira D, Lopes C, Medeiros R. Protective role of the polymorphism CCR2-64I in the progression from squamous intraepithelial lesions to invasive cervical carcinoma. Gynecol Oncol. 2005;96:760-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Catarino R, Matos A, Pinto D, Pereira D, Craveiro R, Vasconcelos A, Lopes C, Medeiros R. Increased risk of cervical cancer associated with cyclin D1 gene A870G polymorphism. Cancer Genet Cytogenet. 2005;160:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Duarte I, Santos A, Sousa H, Catarino R, Pinto D, Matos A, Pereira D, Moutinho J, Canedo P, Machado JC. G-308A TNF-alpha polymorphism is associated with an increased risk of invasive cervical cancer. Biochem Biophys Res Commun. 2005;334:588-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Papafili A, Hill MR, Brull DJ, McAnulty RJ, Marshall RP, Humphries SE, Laurent GJ. Common promoter variant in cyclooxygenase-2 represses gene expression: evidence of role in acute-phase inflammatory response. Arterioscler Thromb Vasc Biol. 2002;22:1631-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 260] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 35. | Hamajima N, Takezaki T, Matsuo K, Saito T, Inoue M, Hirai T, Kato T, Ozeki J, Tajima K. Genotype Frequencies of Cyclooxygenease 2 (COX2) Rare Polymorphisms for Japanese with and without Colorectal Cancer. Asian Pac J Cancer Prev. 2001;2:57-62. [PubMed] |
| 36. | Koh WP, Yuan JM, van den Berg D, Lee HP, Yu MC. Interaction between cyclooxygenase-2 gene polymorphism and dietary n-6 polyunsaturated fatty acids on colon cancer risk: the Singapore Chinese Health Study. Br J Cancer. 2004;90:1760-1764. [PubMed] |
| 37. | Goodman JE, Bowman ED, Chanock SJ, Alberg AJ, Harris CC. Arachidonate lipoxygenase (ALOX) and cyclooxygenase (COX) polymorphisms and colon cancer risk. Carcinogenesis. 2004;25:2467-2472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Ulrich CM, Whitton J, Yu JH, Sibert J, Sparks R, Potter JD, Bigler J. PTGS2 (COX-2) -765G > C promoter variant reduces risk of colorectal adenoma among nonusers of nonsteroidal anti-inflammatory drugs. Cancer Epidemiol Biomarkers Prev. 2005;14:616-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Brosens LA, Iacobuzio-Donahue CA, Keller JJ, Hustinx SR, Carvalho R, Morsink FH, Hylind LM, Offerhaus GJ, Giardiello FM, Goggins M. Increased cyclooxygenase-2 expression in duodenal compared with colonic tissues in familial adenomatous polyposis and relationship to the -765G > C COX-2 polymorphism. Clin Cancer Res. 2005;11:4090-4096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Kang S, Kim YB, Kim MH, Yoon KS, Kim JW, Park NH, Song YS, Kang D, Yoo KY, Kang SB. Polymorphism in the nuclear factor kappa-B binding promoter region of cyclooxygenase-2 is associated with an increased risk of bladder cancer. Cancer Lett. 2005;217:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Siezen CL, van Leeuwen AI, Kram NR, Luken ME, van Kranen HJ, Kampman E. Colorectal adenoma risk is modified by the interplay between polymorphisms in arachidonic acid pathway genes and fish consumption. Carcinogenesis. 2005;26:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Zhang X, Miao X, Tan W, Ning B, Liu Z, Hong Y, Song W, Guo Y, Zhang X, Shen Y. Identification of functional genetic variants in cyclooxygenase-2 and their association with risk of esophageal cancer. Gastroenterology. 2005;129:565-576. [PubMed] |
| 43. | Szczeklik W, Sanak M, Szczeklik A. Functional effects and gender association of COX-2 gene polymorphism G-765C in bronchial asthma. J Allergy Clin Immunol. 2004;114:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 44. | Cipollone F, Toniato E, Martinotti S, Fazia M, Iezzi A, Cuccurullo C, Pini B, Ursi S, Vitullo G, Averna M. A polymorphism in the cyclooxygenase 2 gene as an inherited protective factor against myocardial infarction and stroke. JAMA. 2004;291:2221-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 45. | Shi J, Misso NL, Duffy DL, Thompson PJ, Kedda MA. A functional polymorphism in the promoter region of the cyclooxygenase-2 gene is not associated with asthma and atopy in an Australian population. Clin Exp Allergy. 2004;34:1714-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Dinis-Ribeiro M, Lopes C, da Costa-Pereira A, Guilherme M, Barbosa J, Lomba-Viana H, Silva R, Moreira-Dias L. A follow up model for patients with atrophic chronic gastritis and intestinal metaplasia. J Clin Pathol. 2004;57:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Müllenbach R, Lagoda PJ, Welter C. An efficient salt-chloroform extraction of DNA from blood and tissues. Trends Genet. 1989;5:391. [PubMed] |
| 48. | Sun WH, Yu Q, Shen H, Ou XL, Cao DZ, Yu T, Qian C, Zhu F, Sun YL, Fu XL. Roles of Helicobacter pylori infection and cyclooxygenase-2 expression in gastric carcinogenesis. World J Gastroenterol. 2004;10:2809-2813. [PubMed] |
| 49. | Panguluri RC, Long LO, Chen W, Wang S, Coulibaly A, Ukoli F, Jackson A, Weinrich S, Ahaghotu C, Isaacs W. COX-2 gene promoter haplotypes and prostate cancer risk. Carcinogenesis. 2004;25:961-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 50. | Kovesdi I, Reichel R, Nevins JR. Role of an adenovirus E2 promoter binding factor in E1A-mediated coordinate gene control. Proc Natl Acad Sci USA. 1987;84:2180-2184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 217] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 51. | Davis JN, McCabe MT, Hayward SW, Park JM, Day ML. Disruption of Rb/E2F pathway results in increased cyclooxygenase-2 expression and activity in prostate epithelial cells. Cancer Res. 2005;65:3633-3642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-362. [PubMed] |
| 53. | Cambria-Kiely JA, Gandhi PJ. Aspirin resistance and genetic polymorphisms. J Thromb Thrombolysis. 2002;14:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 54. | Gonzalez-Conejero R, Rivera J, Corral J, Acuña C, Guerrero JA, Vicente V. Biological assessment of aspirin efficacy on healthy individuals: heterogeneous response or aspirin failure. Stroke. 2005;36:276-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |