Published online Sep 7, 2006. doi: 10.3748/wjg.v12.i33.5419
Revised: June 8, 2006
Accepted: June 16, 2006
Published online: September 7, 2006
Primary squamous cell carcinoma (SCC) of the liver is rare. Totally nine such cases have been reported in the literature. Primary SCC of the liver has been reported to be associated with hepatic teratoma, hepatic cyst, or hepatolithiasis. Complete remission of poorly differentiated SCC of the liver could be achieved by systemic chemotherapy followed by surgery or remarkably respond to hepatic arterial injection of low dose chemotherapeutic drugs. Here we report the first case of primary SCC of the liver presenting as a solid tumor and receiving successful hepatic resection with 9-mo disease free survival.
- Citation: Lee HL, Liu YY, Yeh CN, Chiang KC, Chen TC, Jan YY. Primary squamous cell carcinoma of the liver: A successful surgically treated case. World J Gastroenterol 2006; 12(33): 5419-5421
- URL: https://www.wjgnet.com/1007-9327/full/v12/i33/5419.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i33.5419
Primary squamous cell carcinoma (SCC) of the liver is rare and reported sporadically. It has been reported to be associated with hepatic teratoma, hepatic cysts, or hepatolithiasis[1-3]. Boscolo showed that a case of complete remission of poorly differentiated SCC of the liver could be achieved by systemic chemotherapy followed by surgery[4]. Kaji demonstrated that a case of primary SCC of the liver could remarkably respond to hepatic arterial injection of low dose chemotherapeutic drugs[5]. To our knowledge, a total of nine cases of primary SCC of the liver have been reported and here we report a case presenting as a solid tumor and receiving successful hepatic resection with disease free survival for 9 mo[1-9].
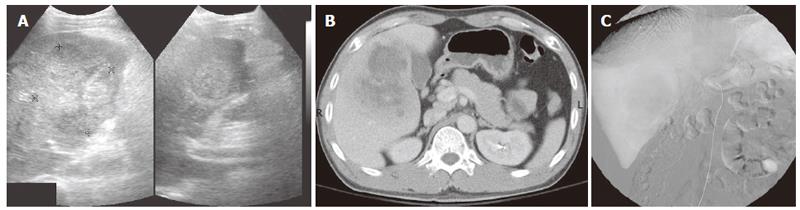
A 40-year-old man presented with right upper quadrant (RUQ) pain for several weeks before admission in September 2005. He did not drink alcohol, smoke, or have hepatitis or a history of surgery. On admission, he had a fever and temperature elevated to 38°C. There was no vomiting, jaundice, dysuria, chills, or abdominal distention. Physical examination revealed tenderness over the RUQ area without a palpable abdominal mass. The following laboratory data were recorded: hemoglobin 13.5 g/dL; white blood cell count 14 600/mm3; platelet: 421 000/μL; prothrombin time (test/normal control): 13.4/11.2 s; international normalized ratio (INR): 1.19; albumin: 3.8 g/dL; direct bilirubin: 0.3 mg/dL; total bilirubin: 0.6 mg/dL; aspartate aminotransferase (AST): 28 IU/L; alanine aminotransferase (ALT): 5 IU/L; alkaline phosphatase (ALP): 163 U/L; blood urea nitrogen: 16 mg/dL; creatinine: 1.2 mg/dL; sodium: 131 meq/L; potassium: 4.1 meq/L; calcium: 10.5 mg/dL; C-reactive protein: 172.78 mg/L. The serum level of carcinoembryonic antigen (CEA) (< 5 ng/mL), alpha-fetoprotein (AFP) (< 15 ng/mL), and carbohydrate antigen 19-9 (CA 19-9) (< 37 U/mL) were 3.06 ng/mL, < 3 ng/ mL, and 4.78, respectively. The plasma retention rate of indocyanine green (ICG) at 15 min was 0.21%. Subsequent abdominal ultrasonography (US) showed a mixed echoic mass about 7.8 cm occupying S5 of the right lobe of the liver (Figure 1A). The abdominal computed tomography (CT) showed a 9.5 cm × 7.0 cm irregular mass with inhomogeneous density and mild delayed enhancement in the central zone of the tumor near the gallbladder, at S5 of right lobe of the liver (Figure 1B). Angiography showed a large tumor with neovascularity and stretching the feeding arteries, located at S5 of right lobe of the liver. Diagnosis of a liver mass with hypervascularity at S5 of the right lobe of the liver was considered (Figure 1C).
In October 2005, we performed extended right lobectomy and cholecystectomy to remove the liver mass. Intra-operative finding showed non-cirrhotic liver with a 10 cm × 8 cm hard and white tumor mass with central necrosis located at S4, 5 and 6 (mainly S5) with no tumor rupture and no hemoperitoneum. The resection margin was 2.0 cm in width.
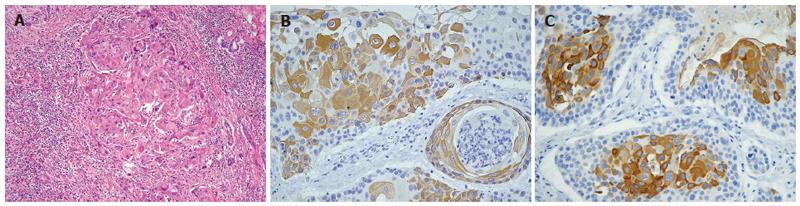
Histopathological examination showed moderately differentiated SCC of the liver composed of squamous cells with keratinization (Figure 2A). An imminohisto-chemical study revealed positive for cytokeratin (CK) 10, CK14, CK19 and CEA. The occasional positivity for CK18 but negativity for thyroid transcription factor-I (TTF-1) indicated that it could be a primary SCC of the liver (Figure 2B, 2C). Subsequent panendoscopy showed negative findings for the esophagus. Chest CT revealed no mass over the lung field and ENT survey showed a negative finding in the oral cavity and nasopharynx. Postoperative course was uneventful and no tumor recurrence and distant metastasis developed during a 9-mo follow-up period.
Primary SCC of the liver is very rare. Although the carcinogenesis has been proposed of tumor transformation from chronic inflammation of the biliary epithelium or metaplastic and subsequent neoplastic transformation of pre-existing cysts of the liver[1-3,8], as shown in this case, the true mechanism is still unknown.
Here, we reported the first case of SCC of the liver, presenting with a solitary solid tumor without parasitic infection and curatively treated by hepatic resection successfully. In this case, the pathology report showed moderately differentiated SCC composed of squamous cells with keratinization. The positive staining of an acidic CK 14 indicated basal cells of keratinized squamous epithelium origin of the cancer cell. The strong, diffuse expression of biliary CK 19 confirmed the bile ductular ontogeny of the neoplastic cells. Thus tumor transformation from chronic inflammation of the biliary epithelium might explain its origin. Because TTF-I, an indicator of small cell carcinoma of the lung or thyroid is negative, metastatic lung or thyroid cancer to the liver could be ruled out. Clinically, panendoscopy, chest CT, and ENT examination revealed negative findings in the case. Taken together, the tumor could be a primary SCC of the liver[10,11].
The prognosis of primary SCC of the liver is dismal with overall survival less than one year[7-9], because the tumor is usually diagnosed late. Complete remission of poorly differentiated SCC after systemic chemotherapy (cisplatin and 5-fluorouracil) and surgery has been reported[4]. Hepatic arterial injection of low dose anti-cancer drugs (cis-diaminedichloroplatinum) (CDDP) and 250 mg of 5-fluorouracil (5-FU)) could achieve remarkably good response[5]. As shown in the case, the postoperative course was smooth with disease free survival for 9 mo. Aggressive and meticulous follow up is needed. If tumor recurrence is detected, re-hepatectomy, systemic chemotherapy (cisplatin and 5-fluorouracil), or hepatic arterial infusion of low dose chemotherapy could be considered[4,5].
In summary, we describe the first case of a primary SCC of the liver presenting as a solitary solid tumor, and non-parasitic infection, successfully treated by surgical resection. Postoperative course was uneventful with 9-mo disease free survival. Aggressive and meticulous follow-up is warranted.
S- Editor Wang J L- Editor Zhu LH E- Editor Bai SH
| 1. | Shinagawa T, Tadokoro M, Takagi M, Yasuda R, Adachi Y, Komoriyama H, Yamaguchi S. Primary squamous cell carcinoma of the liver: a case report. Acta Cytol. 1996;40:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Banbury J, Conlon KC, Ghossein R, Brennan MF. Primary squamous cell carcinoma within a solitary nonparasitic hepatic cyst. J Surg Oncol. 1994;57:210-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Nieweg O, Slooff MJ, Grond J. A case of primary squamous cell carcinoma of the liver arising in a solitary cyst. HPB Surg. 1992;5:203-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Boscolo G, Jirillo A, Da Pian P. Complete remission of poorly differentiated squamous liver carcinoma after systemic chemotherapy and surgery. A case report. Tumori. 2005;91:71-72. [PubMed] |
| 5. | Kaji R, Sasaki N, Tateishi I, Nagata E, Okabe Y, Yoshida T, Sata M, Ueno T. A case report of primary hepatic squamous cell carcinoma that remarkably responded to low dose arterial injection of anti-cancer drugs. Kurume Med J. 2003;50:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Roediger WE, Dymock RB. Primary squamous carcinoma of the liver: clinical and histopathological features. Aust N Z J Surg. 1991;61:720-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Yagi H, Ueda M, Kawachi S, Tanabe M, Aiura K, Wakabayashi G, Shimazu M, Sakamoto M, Kitajima M. Squamous cell carcinoma of the liver originating from non-parasitic cysts after a 15 year follow-up. Eur J Gastroenterol Hepatol. 2004;16:1051-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Clements D, Newman P, Etherington R, Lawrie BW, Rhodes J. Squamous carcinoma in the liver. Gut. 1990;31:1333-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Bloustein PA, Silverberg SG. Squamous cell carcinoma originating in an hepatic cyst. Case report with a review of the hepatic cyst-carcinoma association. Cancer. 1976;38:2002-2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Alison MR, Lovell MJ. Liver cancer: the role of stem cells. Cell Prolif. 2005;38:407-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Tsuneyama K, Kaizaki Y, Doden K, Kidani E, Harada K, Sasaki M, Nakanuma Y. Combined hepatocellular and cholangiocarcinoma with marked squamous cell carcinoma components arising in non-cirrhotic liver. Pathol Int. 2003;53:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |