Published online Sep 7, 2006. doi: 10.3748/wjg.v12.i33.5331
Revised: February 28, 2004
Accepted: March 2, 2004
Published online: September 7, 2006
AIM: To study the selective killing of human umbilical vein endothelial cells (HUVECs) by a double suicide gene under the regulation of a kinase domain insert containing receptor (KDR) promoter and mediated by an adenoviral gene vector.
METHODS: Human KDR promoter was cloned by polymerase chain reaction (PCR), and two recombinant adenoviral plasmids pAdKDR-CdglyTK, pAdCMV-CDglyTK were constructed according to a two-step transformation protocol. These two newly constructed plasmids were then transfected into 293 packaging cells to grow adenovirus, which were further multiplied and purified. HUVECs and LoVo cells were infected with either of the two resultant recombinant adenoviruses (AdKDR-CDglyTK and AdCMV-CDglyTK) respectively, and the infection rates were estimated by detection of green fluorescent protein (GFP) expression. Infected cells were cultured in culture media containing different concentrations of 5-fluorocytosine (5-FC) and ganciclovir (GCV), and the killing effects were measured.
RESULTS: The two recombinant adenoviral plasmids pAdKDR-CdglyTK, pAdCMV-CDglyTK were successfully constructed and transfected into 293 cells. The resultant recombinant adenoviruses infected cells caused similar infection rates; and the infected cells exhibited different sensitivity to the prodrugs: HUVECs infected with AdCMV-CDglyTK and LoVo cells infected with AdCMV-CDglyTK were highly sensitive to the prodrugs, and HUVECs infected with AdKDR-CDglyTK were similarly sensitive but significantly more sensitive than the LoVo cells infected with AdKDR-CdglyTK (P < 0.001).
CONCLUSION: Selective killing of HUVECs may be achieved by gene transfer of double suicide gene under the regulation of the KDR promoter. This finding may provide an optional way to target gene therapy of malignant tumors by abrogation of tumor blood vessels.
- Citation: Yang WY, Huang ZH, Lin LJ, Li Z, Yu JL, Song HJ, Qian Y, Che XY. Kinase domain insert containing receptor promoter controlled suicide gene system selectively kills human umbilical vein endothelial cells. World J Gastroenterol 2006; 12(33): 5331-5335
- URL: https://www.wjgnet.com/1007-9327/full/v12/i33/5331.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i33.5331
Gene therapy is a novel technology leading to improved treatments of some types of cancer[1-3]. On the other hand, anti-angiogenic therapy has also been proven to be a rational approach in the treatment of solid tumors also[4-9]. Selective delivery of the suicide gene into tumor endothelial cells and target expression of it to abrogate tumor vasculature may be a good way for tumor therapy.
A suicide gene is a gene encoding an enzyme that converts nontoxic prodrugs into toxic forms. The herpes simplex virus-thymidine kinase (TK) gene is one of the most widely studied suicide genes[10], and the E. coli cytosine deaminase (CD) gene is another widely studied one[11-13]. However, results of numerous investigations aimed at eradicating tumors employing either CD or TK demonstrate their limitations. Whether the limitations stem from unfavorable pharmacokinetics, loss of transgene expression or biochemical resistance is not clear. Nevertheless, fusion gene of CD and TK shows exciting superiority in many studies[14-17].
The adenovirus vector system has many advantages[18-20]. First, adenovirus vectors can be prepared at much higher titers than retroviral vectors and have a high efficiency of gene transfer regardless of the proliferative states of the tissues whereas retroviral vectors insert their genes only into dividing cells. Second, adenovirus genomes usually do not integrate into the host cell chromosome, and the level of therapeutic gene expression is very high. Human umbilical vein endothelial cells (HUVECs) are primary cells that can be used to investigate the mechanisms underlying the role of endothelial cells[21]. The kinase domain insert containing the receptor (KDR) gene is strictly expressed only in vascular endothelium cells. The activity of the KDR promoter in endothelial cells is similar to that of the potent SV40 promoter/enhancer and that this high level activity is specific to endothelial cells[22].
We constructed two recombinant adenoviruses to transfer the double suicide gene under the KDR promoter or the CMV promoter into HUVECs respectively, and LoVo cells (a cell system of colon carcinoma) were used as the control. Prodrug sensitive experiments were performed to evaluate the killing effect of the fusion double suicide gene under the regulation of the KDR promoter, mediated by the adenovirus vector on HUVEC cells.
Shutter plasmid pAdtrack, pAdtrack-CMV, adenoviral backbone plasmid pAdEasy-1 and E.coli BJ5183 were provided by Dr. Belt Vogelstein at Johns Hopkins Oncology Center, Howard Hughes Institute of Medicine. pMD18-T vector was purchased from TaKaRa Biotechnology (Dalian) Co. Ltd. 293 cells, and HUVECs were obtained from American Type Culture Collection (ATCC). All sorts of exonuclease, T4 DNA ligase, and Taq DNA polymerase were purchased from New England Biolabs Co. DMEM, fetal bovine serum (FBS), transfection reagents, and LipofectAMINE2000 were products of Gibco Co. Primers of KDR promoter gene, CD gene, TK gene were synthesized and sequenced by Sangon Biotechnology (Shanghai) Co., Ltd.
KDR promoter genes (including the minimus core of the gene sequence -226~+268) were generated by PCR using human blood genome as the template (the upstream primer sequence: 5’-GGAAGATCTAGTTGCTCAGCGCCCGTTAC-3’, the downstream primer sequence: 5’-CCCAAGCTTGGCGAAATGCCCAGAACTCG-3’). The resulting fragment containing Bgl at the 5’ end, and HindIII restriction sites at the 3’ end respectively were linked to pMD18-T vector to generate pMD-18KDR. Another two PCR protocols were performed to generate CD and TK gene sequences(the upstream primer sequence of CD: 5’-AAGCTTAGGCTAGCAATGTCGAATAACGCT-3’, the downstream primer sequence: 5’-GGATCCTCCACGTTTGTAATCGATGGCTTC-3’; the upstream primer sequence of TK: 5’-GGATCCGGCGGGGGCGGTGGAGGAGGGGGTATGGCTTCGTAC-3’, the downstream primer sequence of TK: 5’-TCTAGATTAGTTAGCCTCCCCCATCTC-3’. The chromosome DNA of E.coli JM109 was used as template for CD gene amplification, and plasmid pREP8-TK for TK gene). According to the protocols, the initiation codon of the resulting CD gene was converted to ATG, termination codon of TAG to GGA which is to code for glycine, and its 5’ end was to have BglII, and 3’ end to have HindIII restriction sites respectively. The two amplified fragments were subcloned into pcDNA3 vectors to generate pcDNA3-CDglyTK.
KDRs were removed from pMD-18KDR by BglII, HindIII and subcloned into pcDNA3-CDglyTK to generate pcDNA3-KDR-CDglyTK, which was digested with BglII and PvuII to get KDR-CDglyTK fragment. The resulting KDR-CDglyTK fragments were subcloned into pAdtrack to generate pAdtrackKDR-CDglyTK. CDglyTK fragement from pcDNA3-CDglyTK (HindIII, XbaI ) was subcloned into pAdtrackCMV to construct pAdtrackCMV-CdglyTK.
pAdEasey-1 plasmid was transformed into E.coli BJ5183, followed by growing of transformants on LB agar plates containing ampicillin and streptomycin. The transformed bacterium was named “AdEasey-1 bacteria”.
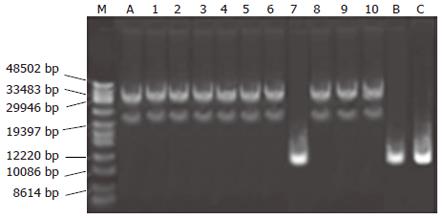
pAdtrackKDR-CDglyTK was linearized and transformed into AdEasey-1 bacteria. Transformants were selected on LB agar plates containing 25 μg/mL kanamycin. Plasmid DNA was prepared from individual colonies, and agarose gel electrophoresis was performed. The correct recombinant pAdKDR-CDglyTK could be clearly identified by size, as only the 11.2-kb pAdtrackKDR-CDglyTK plasmid and the 37-kb pAdKDR-CDglyTK were selectable by kanamycin resistance.
The same protocol was performed to transfer pAdtrackCMV-CDglyTK into AdEasey-1 bacteria to recombine with pAdCMV-CDglyTK.
Both pAdKDR-CDglyTK and pAdtrackCMV-CDglyTK were transferred into 293 cells mediated by LipofectAMINE2000 vector. Their further propagation was visualized under fluorescence microscope by GFP expression of transgene, and ultracentrifugation in CsCl gradient was performed to purify the viruses, and the titration of AdKDR-CDglyTK and AdtrackCMV-CDglyTK was measured by plaque formation assay.
The resultant recombinant viruses were boiled to be used as templates. PCR protocols were performed either to ensure the presence of CDglyTK gene in the viruses, in which upstream primer sequence of CD and downstream primer sequence of TK were used, or to ensure the presence of the KDR promoter gene in AdKDR-CdglyTK. Primers of KDR promoter were used.
HUVECs and LoVo cells, obtained from ATCC, were maintained in calorstart of 37°C, with 5% CO2 and in Dulbecco’s modified Eagle’s medium(DMEM) supplemented with 10% fetal bovine serum (FBS). Cells (2 × 105/well, 6-well plate) were infected with AdKDR-CDglyTK or AdCMV-CDglyTK at different multiplicity of infection (MOI). Percentages of cells expressing GFP were counted under fluorescence microscope within 3 d.
HUVECs or LoVo cells (1 × 104 cells/well, 96-well plate, inoculated one day before) were infected with AdKDR-CDglyTK or AdCMV-CDglyTK at MOI of 100, 16 h later. The medium was removed and replaced with fresh medium with different concentrations of 5-FC and/or GCV. Cells were cultured in the presence of prodrugs for 72 h. Cell viability was determined by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay according to the manufacturer’s protocol.
Statistical analyses were made by ANOVA and LSD test. The values were calculated as means ± SD. P < 0.05 was considered statistically significant.
The products of PCR amplification or constructed KDR promoter gene, CD and TK genes and CDglyTK gene were sequenced and verified by Sangon Biotechnology (Shanghai) Co., Ltd.
Newly constructed pAdtrackKDR-CDglyTK and pAdtrackCMV-CDglyTK were identified by restriction analysis and transferred into AdEasier-1 bacteria. Recombinant bacteria were selected by kanamycin. Figure 1 is the map of plasmids from 10 clones of transferred bacteria selected by kanamycin. Nine of them were correctly recombined, with a correction rate of 90% (9/10).
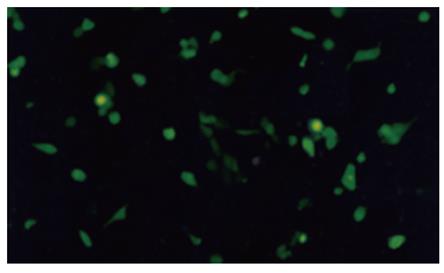
Three days after transferring pAdKDR-CDglyTK and pAdCMV-CDglyTK into 293 cells, we found that most transferred 293 cells expressed GFP (Figure 2). The titer of the purified virus after being sufficiently propagated was 3.5 × 1012 pfu/L. No difference between AdKDR-CDglyTK and AdCMV-CdglyTK was observed.
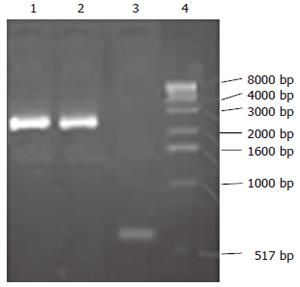
The products of the PCR using the recombinant viruses as templates were proven to be correct by size comparison (Figure 3), with KDR promoter of 580-bp and CdglyTK of 2.5-bp.
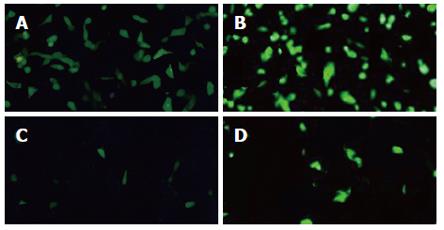
HUVECs and LoVo cells infected with AdKDR-CDglyTK or AdCMV-CDglyTK at different MOI were observed 3 d post-infection (Figure 4). It was indicated that cell infection rate increased with the increase of MOI of the virus: when MOI = 1, only a few cells expressed GFP; when MOI = 100, most cells expressed GFP; when MOI = 200, almost all cells expressed GFP. There was no difference between the two recombinant viruses in infecting HUVECs and LoVo cells.
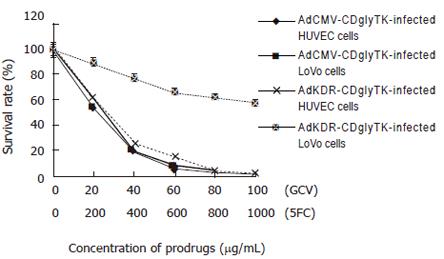
HUVECs and LoVo cells infected with AdKDR-CDglyTK or AdCMV-CDglyTK at MOI of 100 were maintained in culture medium of different concentration of GCV and 5-FC for 3 d, and the survival rates were measured (Figure 5). Both AdCMV-CDglyTK-infected HUVECs and AdCMV-CDglyTK-infected LoVo cells were highly sensitive to the prodrugs. More than 80% of both transgene cells were killed when they were treated with 40 μg/mL GCV + 400 μg/mL 5-FC; almost all of both types of cells were killed when treated with 100 μg/mL GCV + 1000 μg/mL 5-FC. The sensitivities of these two transgeneic cells were of no much difference (P = 0.518). AdKDR-CDglyTK-infected HUVECs were highly sensitive to the prodrugs, similarly (P > 0.2); however, AdKDR-CDglyTK-infected LoVo cells were far less sensitive to the prodrugs (P < 0.001). There was still 57.2% survival of cells 3 d after treatment with 100 μg/mL GCV + 1000 μg/mL 5-FC.
A blood supply is required for a tumor to progress in excess of 1-2 mm3[23,24]. Recent data suggest that tumor cells can co-opt surrounding vasculature from an even smaller size in certain tumor types[25]. The expanding growth of a primary tumor is associated with neo-angiogenesis and vessel maturation of the tumor. Abrogation of tumor blood vessel should lead to the eradication or suppression of solid tumor. Moreover, Folkman proposed that the prevention of tumor vascularization essentially stops neoplastic invasion, which may lead to the ablation of metastatic disease[26]. The rapid growth of a tumor results in hypoxia[27] which induces the production of pro-angiogenic factors such as vascular endothelial growth factor (VEGF)[28]. VEGF is a potent and specific mitogen for endothelial cells. It plays a major role in angiogenesis and vasculogenesis. KDR and Flt-1 are the two receptor tyrosine kinases that regulate the actions of VEGF and are expressed in endothelial cells. KDR is critically involved in the regulation of angiogenesis, both in the developing and adult animals[29]. The vascular endothelial cell is renewed at a low speed in normal conditions, and its KDR expression level is very low, while tumor vascular endothelial cells proliferate quickly and with a KDR expression level 500 times higher than that of vascular endothelial cells of normal tissues[30]. Therefore, it is possible to realize targeted expression of therapeutic genes in tumor vascular endothelial cells by transcriptional regulation of the KDR promoter, thus markedly reducing the toxicity and side effects of gene therapy targeting vascular endothelial cells of tumors.
In our experiments, we constructed two replication-incompetent recombinant adenoviral vectors with the AdEasy system in a “two-step transformation protocol” to transfer the KDR-promoter-controlled/CMV-promoter-controlled double suicide gene into HUVEC/LoVo cells. The results indicated that “two-step transformation protocol” is a convenient and efficient way to generate adenoviral vectors. The resultant adenoviruses infected HUVECs with a great efficiency and the transgenes were efficiently expressed, which was delineated by the reporter gene GFP as it was expressed in almost all HUVECs when they were infected with the virus at MOI of 200. The subsequent prodrug sensitivity experiments demonstrated that this high level expression was strong enough for cell killing in vitro. The prodrug sensitivity experiment also demonstrated that CMV-promoter-controlled double suicide gene/prodrugs system unselectively killed both HUVECs and LoVo cells; however, the KDR-promoter-controlled double suicide gene/ prodrugs system displayed a targeted killing effect on HUVECs: treated with 40 μg/mL GCV + 400 μg/mL 5-FC, 74.8% KDR-CDglyTK-transferred HUVECs were killed while 77.6% KDR-CDglyTK-transferred LoVo cells were alive; treated with 100 μg/mL GCV + 1000 μg/mL 5-FC, 98.5% KDR-CDglyTK-transferred HUVECs were killed while the killing rate of KDR-CDglyTK-transferred LoVo cells was 42.8%. All these come to an exciting conclusion that: the KDR-promoter-controlled double suicide gene/prodrugs system bears a selectively killing effect on HUVEC. This finding may provide an optional way for targeting gene therapy of malignant disease by abrogation of tumor blood vessels.
S- Editor Wang J L- Editor Zhu LH E- Editor Ma WH
| 1. | Zeng M, Smith SK, Siegel F, Shi Z, Van Kampen KR, Elmets CA, Tang DC. AdEasy system made easier by selecting the viral backbone plasmid preceding homologous recombination. Biotechniques. 2001;31:260-262. [PubMed] |
| 2. | He Y, Zhou J, Wu JS, Dou KF. Inhibitory effects of EGFR antisense oligodeox ynucleotide in human colorectal cancer cell line. World J Gastroenterol. 2000;6:747-749. [PubMed] |
| 3. | Fan YF, Huang ZH. Progress in the studies of gene therapy for colorectal cancer. Shijie Huaren Xiaohua Zazhi. 2001;9:427-430. |
| 4. | Pastorino F, Brignole C, Marimpietri D, Cilli M, Gambini C, Ribatti D, Longhi R, Allen TM, Corti A, Ponzoni M. Vascular damage and anti-angiogenic effects of tumor vessel-targeted liposomal chemotherapy. Cancer Res. 2003;63:7400-7409. [PubMed] |
| 5. | Mentlein R, Held-Feindt J. Angiogenesis factors in gliomas: a new key to tumour therapy. Naturwissenschaften. 2003;90:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Li Y, Jin Y, Chen H, Jie G, Tobelem G, Caen JP, Han ZC. Suppression of tumor growth by viral vector-mediated gene transfer of N-terminal truncated platelet factor 4. Cancer Biother Radiopharm. 2003;18:829-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Lamszus K, Kunkel P, Westphal M. Invasion as limitation to anti-angiogenic glioma therapy. Acta Neurochir Suppl. 2003;88:169-177. [PubMed] |
| 8. | Yang SH, Lin JK, Chen WS, Chiu JH. Anti-angiogenic effect of silymarin on colon cancer LoVo cell line. J Surg Res. 2003;113:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Longo R, Sarmiento R, Fanelli M, Capaccetti B, Gattuso D, Gasparini G. Anti-angiogenic therapy: rationale, challenges and clinical studies. Angiogenesis. 2002;5:237-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Jiao LR, Havlik R, Nicholls J, Jensen SL, Habib NA. Suicide gene therapy in liver tumors. Methods Mol Med. 2004;90:433-450. [PubMed] |
| 11. | Miyagi T, Koshida K, Hori O, Konaka H, Katoh H, Kitagawa Y, Mizokami A, Egawa M, Ogawa S, Hamada H. Gene therapy for prostate cancer using the cytosine deaminase/uracil phosphoribosyltransferase suicide system. J Gene Med. 2003;5:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Brown NL, Lemoine NR. Clinical trials with GDEPT: cytosine deaminase and 5-fluorocytosine. Methods Mol Med. 2004;90:451-457. [PubMed] |
| 13. | Porosnicu M, Mian A, Barber GN. The oncolytic effect of recombinant vesicular stomatitis virus is enhanced by expression of the fusion cytosine deaminase/uracil phosphoribosyltransferase suicide gene. Cancer Res. 2003;63:8366-8376. [PubMed] |
| 14. | Rogulski KR, Kim JH, Kim SH, Freytag SO. Glioma cells transduced with an Escherichia coli CD/HSV-1 TK fusion gene exhibit enhanced metabolic suicide and radiosensitivity. Hum Gene Ther. 1997;8:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Jiang YR, Ma DX, Chen XL, Liu CS. [Killing effect of double suicide genes mediated by lentivirus on lymphoma cells]. Ai Zheng. 2003;22:916-921. [PubMed] |
| 16. | Li XJ, Wang KM, Xu Y, Wang ZH, Xia AD, Chen SS, Qian GX. [Ionizing radiation-regulated killing of human hepatoma cells by liposome-mediated CDglyTK gene delivery]. Shengwu Huaxue Yu Shengwu Wuli Xuebao (Shanghai). 2003;35:64-70. [PubMed] |
| 17. | Corban-Wilhelm H, Becker G, Bauder-Wüst U, Greulich D, Debus J. Cytosine deaminase versus thymidine kinase: a comparison of the antitumor activity. Clin Exp Med. 2003;3:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Hoganson DK, Batra RK, Olsen JC, Boucher RC. Comparison of the effects of three different toxin genes and their levels of expression on cell growth and bystander effect in lung adenocarcinoma. Cancer Res. 1996;56:1315-1323. [PubMed] |
| 19. | Kenneth RR, Jae HK, Sang HK, Svend OF. Glioma cells transduced with an Escherichia coli CD/HSV-1TK fushion gene exhibit enhanced metabolic suicide and radiosensitivity. Human Gene Therapy. 1997;8:73-85. |
| 20. | Trinh QT, Austin EA, Murray DM, Knick VC, Huber BE. Enzyme/prodrug gene therapy: comparison of cytosine deaminase/5-fluorocytosine versus thymidine kinase/ganciclovir enzyme/prodrug systems in a human colorectal carcinoma cell line. Cancer Res. 1995;55:4808-4812. [PubMed] |
| 21. | Ear T, Giguère P, Fleury A, Stankova J, Payet MD, Dupuis G. High efficiency transient transfection of genes in human umbilical vein endothelial cells by electroporation. J Immunol Methods. 2001;257:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Patterson C, Perrella MA, Hsieh CM, Yoshizumi M, Lee ME, Haber E. Cloning and functional analysis of the promoter for KDR/flk-1, a receptor for vascular endothelial growth factor. J Biol Chem. 1995;270:23111-23118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 138] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Folkman J. What is the evidence that tumors are angiogenesis dependent. J Natl Cancer Inst. 1990;82:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3254] [Cited by in RCA: 3194] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 24. | Jakóbisiak M, Lasek W, Gołab J. Natural mechanisms protecting against cancer. Immunol Lett. 2003;90:103-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1620] [Cited by in RCA: 1528] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 26. | Gross J, Azizkhan RG, Biswas C, Bruns RR, Hsieh DS, Folkman J. Inhibition of tumor growth, vascularization, and collagenolysis in the rabbit cornea by medroxyprogesterone. Proc Natl Acad Sci USA. 1981;78:1176-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 80] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Gee MS, Koch CJ, Evans SM, Jenkins WT, Pletcher CH Jr, Moore JS, Koblish HK, Lee J, Lord EM, Trinchieri G. Hypoxia-mediated apoptosis from angiogenesis inhibition underlies tumor control by recombinant interleukin 12. Cancer Res. 1999;59:4882-4889. [PubMed] |
| 28. | Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3206] [Cited by in RCA: 3224] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 29. | Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem. 1997;272:23659-23667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 537] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 30. | Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992;3:65-71. [PubMed] |