Published online Sep 7, 2006. doi: 10.3748/wjg.v12.i33.5326
Revised: March 28, 2006
Accepted: April 24, 2006
Published online: September 7, 2006
AIM: To compare, in a pig liver transplantation model, the protective effect of UW with that of IGL-1, a high-sodium preservation solution containing polyethylene glycol (PEG) as an oncotic supply.
METHODS: All livers were harvested and grafted orthotopically according to standard techniques. The livers were washed out and preserved for 7 h in IGL-1 (n = 6) or in UW solution (n = 7) at 4°C. In a sham group (n = 4), the livers underwent a 60-min warm ischemia at 37°C. The hepatocellular injury was assessed in organ preservation solution washed out from the graft at the end of ischemic storage (before revascularization), and in serum 2 h after reperfusion and daily for up to 6 d.
RESULTS: Livers preserved in IGL-1 solution released markedly less AST than that preserved in the UW solution before and after revascularization (P < 0.05). Besides, the activity of creatine kinase-BB, a marker of sinusoidal lining cells injury, was higher in the UW group than in the IGL-1 group (P < 0.05). Histological results showed less necrotic regions in livers preserved in IGL-1 solution; however, no difference was observed for inflammation.
CONCLUSION: IGL-1 liquid effectively protects parenchymal and non-parenchymal cells against prese-rvation-reperfusion injuries.
- Citation: Abdennebi HB, Elrassi Z, Scoazec JY, Steghens JP, Ramella-Virieux S, Boillot O. Evaluation of IGL-1 preservation solution using an orthotopic liver transplantation model. World J Gastroenterol 2006; 12(33): 5326-5330
- URL: https://www.wjgnet.com/1007-9327/full/v12/i33/5326.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i33.5326
Reperfusion injury after cold preservation is associated with the occurrence of primary non-function and delayed graft function, which predisposes allografts to acute and chronic rejection[1]. Despite these obstacles, organ transplantation has become a clinical routine for several years. Nevertheless, the need of extended ischemia times and the recruitment of marginal donors indicates that this therapy must be optimized. Consequently, it has become necessary to look for better ways to improve cold storage solutions.
The golden standard liquid for the cold preservation of abdominal organs is the University of Wisconsin (UW) solution. Although largely used and incontestably efficient, its performance is continually questioned[2,3]. It has been reported that performances of this solution are limited by its adjunction of hydroxyethyl starch (HES) that has a hyper-aggregating effect on rat and human red blood cells[4,5], and by its high potassium concentration that could damage cells[6,7].
Our previous works on isolated perfused rat livers and kidneys indicates that polyethylene glycol (PEG-35) could be efficiently substituted for HES in an extracellular-like UW solution[8,9]. The clinical use of this Na-PEG-UW solution, also known as IGL-1, suggests a superiority of IGL-1 compared to standard UW for human kidneys preservation[10]. However, to our knowledge, no studies have been reported for liver transplantation. Therefore, we aimed to evaluate the preservative effect of IGL-1 solution using an orthotopic liver transplantation (OLT) model in pigs, and to compare its effectiveness with that of the UW solution.
Female large white pigs, weighing 25-30 kg, were used as organ donors and recipients. All animals were given humane care and handled in compliance with French regulations. Animals were fasted for 24 h prior to the intervention but had free access to water. All animals received pre-anesthesia with tiletamine (10 mg/kg), zolazepam (7.5 mg/kg) and atropine sulfate (10 μg/kg). After intubation, inhalation anesthesia was maintained by halothane (0.5%-1%), nitrous protoxide and oxygen.
We used a standard technique for the donor operation. Portal vein (PV), infrahepatic vena cava (IHVC) and suprahepatic vena cava (SHVC) were dissected. The hepatic artery was kept in continuity with the celiac trunk, and the abdominal aorta up to the diaphragmatic pillars. Livers were harvested and washed out ex situ with 2 L of chilled preservation solution through PV. The harvested grafts were then cold stored at 4°C for 7 h.
In each recipient animal, a catheter was placed into the aorta to monitor blood pressure during the operation. It has been observed that complete vascular clamping during the anhepatic phase generates severe hypotension, acidosis and hyperkalemia, which may cause the death of the animal[11]. We, therefore, placed a porto-jugular passive bypass (PJB). Just before and during the anhepatic phase, 500 mL of Hesteril solution (60 g/L of HES in 9 g/L of NaCl) was infused into the recipient to maintain a stable hemodynamic condition. The graft was positioned in the hepatic fossa, SHVC and IHVC and PV of the donor were then anastomosed, respectively, to those of the recipient with running sutures of 4/0 Prolene (for SHVC and IHVC) and 5/0 Prolene (for PV). During portal anastomosis, the graft was flushed out by Hesteril at room temperature (200 mL, IHVC was used as a vent), to purge the preservation solution and waste metabolites accumulated throughout the cold storage period. The donor aorta was implanted end-to-side to the recipient infra-renal aorta with running 7/0 Prolene, and finally the billiary continuity was re-established by using the intra-ductal stent. Reperfusion was thus started after finishing the portal anastomosis and completed after the arterial anastomosis. After OLT, no immunosuppression was given to the animals.
Animals were divided into three experimental groups. Livers of the UW group (n = 7) were cold stored in the original Belzer liquid without dexamethasone, insulin and antibiotics. Livers of the IGL-1 group (n = 6) were preserved in the same solution but with inverted concentrations of Na+ and K+ and containing PEG-35 that was substituted for HES (Table 1). A sham group (n = 4) was designed for the purpose of evaluating the effect of warm ischemia throughout OLT. Indeed as for recipients, after complete vascular clamping, a PJB was installed for 60 min. Livers were then rinsed with Hesteril solution and finally PV and IHVC were anastomosed.
| UW | IGL-1 | |
| HES (mmol/L) | 0.25 | – |
| PEG-35 (mmol/L) | – | 0.03 |
| Lactobionic acid (mmol/L) | 100 | 100 |
| Raffinose (mmol/L) | 30 | 30 |
| MgSO4 (mmol/L) | 5 | 5 |
| KH2PO4 (mmol/L) | 25 | 25 |
| Glutathione (mmol/L) | 3 | 3 |
| Adenosine (mmol/L) | 5 | 5 |
| Allopurinol (mmol/L) | 1 | 1 |
| Na+ (mmol/L) | 30 | 125 |
| K+ (mmol/L) | 125 | 30 |
| Osmolality (mOsm/Kg) | 320 | 320 |
| pH | 7.2-7.4 | 7.2-7.4 |
The follow-up was of 6 duration. In order to assess the extent of parenchymal cell damage, the release of AST and ALT in serum was monitored 2 h after reperfusion and then daily for 6 d. Kinetic measurements of enzymes were determined at A340 nm on a Hitachi 747 analyzer. In addition, activities of total creatine kinase (CK) and CK-BB isoenzyme[12] were monitored in the organ preservation solution washed out from the graft at the end of ischemic storage and 2 h after reperfusion. Total CK activity was determined with a commercial kinetic UV reagent (BioMérieux, Charbonnières-les-Bains, France). CK isoenzymes were separated electrophoretically on agarose gels with a Paragon kit (Beckman instruments, France). After electrophoresis, CK isoenzymes were detected under UV light, and gels were scanned with a fluorimetric densitometer (SEBIA, France).
Liver biopsies were performed after cold storage and on the 6th d. In sham animals, the biopsies were performed only on the 6th d. Histology was achieved on 4-μm thick paraffin-embedded sections by using conventional hematoxylin-eosin staining. The analysis focused on hepatocellular necrosis, sinusoidal dilatation and inflammatory cellular infiltration. The severity of the histological lesions were scored from 0 (normal liver) to +++ (maximal damage).
Results were expressed as mean ± SE. Data between groups were compared using the ANOVA test, followed by the student Newman-Keul test. P < 0.05 was considered statistically significant.
All the animals survived for up to 6 d. At time of sacrifice, sites of anastomosis were carefully observed, and all were patent. The groups were comparable with regard to warm and cold ischemia.
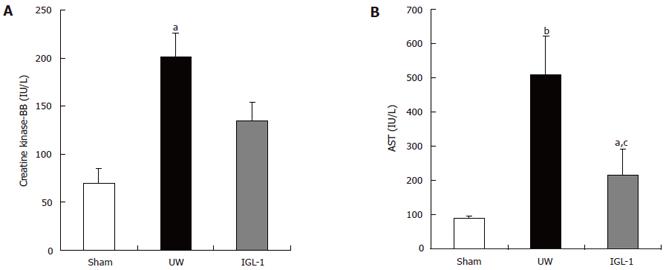
The activities of CK-BB and AST were assessed in the first sample retrieved during initial graft rinsing. The CK-BB isoenzymze was shown to be a marker of sinusoidal lining cells injury[12]. There was no significant difference between sham and IGL-1 groups (Figure 1A). In contrast, livers preserved in UW solution released significantly more CK-BB (P < 0.05) and AST (Figure 1B, P < 0.01) compared to the sham group. Furthermore, the IGL-1-preserved livers released obviously less AST than those preserved in UW liquid (215 ± 78 vs 507 ± 115 IU/L, P < 0.05).
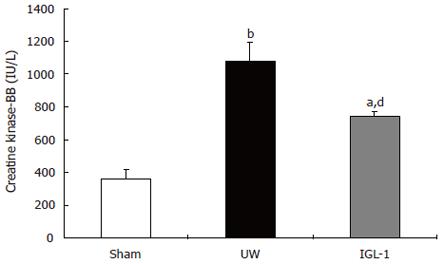
Creatine kinase-BB isoenzyme was also monitored 2 h after reperfusion (Figure 2). The data support the view that the levels of this marker after reperfusion are related to the degree of preservation injury. Indeed, livers preserved in UW released significantly more CK-BB (1081 ± 117 IU/L) than livers of sham (361 ± 59 IU/L, P < 0.01) and those cold stored in IGL-1 (746 ± 29 IU/L, P < 0.01).
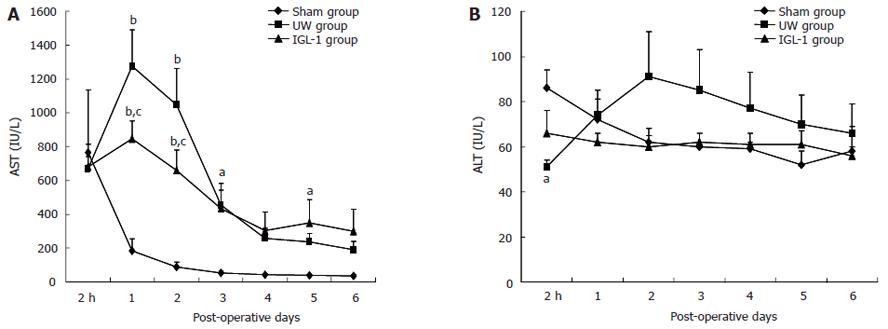
After transplantation, the highest activities of AST (Figure 3A) were reached at d 1-2 in both UW and IGL-1 groups (P < 0.01 vs sham). They were significantly higher in the UW vs IGL-1 group (1275 ± 215 vs 847 ± 103 IU/L at d 1 and 1046 ± 214 vs 658 ± 122 IU/L at d 2, P < 0.05). When livers were preserved in IGL-1 liquid, the activity of ALT (Figure 3B) did not vary at the different times of reperfusion. In contrast, ALT released by livers preserved in the UW peaked at d 2, although there were no statistical differences between the experimental groups.
The histological study (Figure 4 and Table 2) showed no cellular alterations in the sham group. Livers preserved in IGL-1 liquid were normal with mild necrosis. In contrast, the UW group revealed multiple and extensive areas of hepatocyte necrosis.
| Groups | Necrosis | Inflammation |
| UW | +++ | + |
| - | - | |
| - | +++ | |
| - | +++ | |
| ++ | ++ | |
| +++ | ++ | |
| IGL-1 | - | +++ |
| - | +++ | |
| + | + | |
| - | + | |
| - | + |
The prevention of preservation injury is still a subject of interest, the main target of which is the reduction of primary graft non-function or sub-optimal graft function. In this study, the preservative effects of UW and IGL-1 (a PEG-high Na-UW solution) were investigated by using a pig OLT model. The results showed that liver cells integrity was best preserved with IGL-1 liquid.
Our previous studies demonstrated that the simple inversion of Na+ and K+ in the UW (high Na-UW) solution decreased reperfusion injuries and improved rat liver and kidney functions[6,13,14]. With the isolated perfused rat liver model, we showed that PEG substitution for HES in high Na-UW solution (IGL-1) was able to reduce parenchymal and non-parenchymal cell damage[8]. In the autotransplanted pig model, kidneys preserved in IGL-1 solution showed a better function and a significant reduction of MHC classIIexpression, cellular apoptosis and interstitial fibrosis compared to the kidneys preserved in UW liquid[9]. In a preliminary clinical study, we found an important improvement in creatinine clearance and a reduction in cellular apoptosis when IGL-1 was used[10].
It is well known that porcine livers are very sensitive to cold ischemia. In the pig liver transplantation model and after 12 h of cold ischemia, it was reported that all animals died within 24 h of reperfusion, regardless of the cold storage solution used[15]. That is why we preserved livers for 7 h in order to optimize the animal viability.
There is strong evidence that the period of rewarming ischemia after cold preservation and before reperfusion increases hepatocellular injury and affects graft outcome. Devlin et al[16] examined liver enzyme (AST, LDH, purine nucleoside phosphorylase and CK-BB) activities present in the organ preservation solution washed out from the graft at the end of ischemic storage prior to reperfusion. They found a correlation between AST activity level and early post-operative graft viability[16], thereby suggesting that AST is a discriminative marker for post-transplant graft function. In addition, preservation-reperfusion injury is characterized biochemically by high serum AST levels in the early post-operative period[1,17]. In our hands, a statistical difference in AST levels was found between the use of IGL-1 and UW preservation solutions in effluent preservation fluid during graft rinsing. The same results were also observed at 1-2 post-operative days. Although the results of ALT were not significantly different between the three experimental groups, we could notice the absence of any peak after the use of IGL-1. Our biochemical results suggest that IGL-1 preserves parenchymal architecture after cold storage. This is confirmed by the histological study where few necrotic regions were observed in livers cold stored in IGL-1.
Numerous previous studies demonstrated the susceptibility and functional consequences of sinusoidal cells to hypothermic and reperfusion injury. Maintenance of endothelial cells integrity and viability is, therefore, of primary importance during organ transplantation. During the last few years, preservation injury and especially the endothelial cells injury occurring during liver preservation have been described to be due to apoptosis[18-20]. This process is an early event after reperfusion, since it was observed that 3 h after rewarming, almost all cultured liver endothelial cells lost viability[21]. One of the common parameters used in the assessment of sinusoidal cell viability is the release of CK-BB isoenzyme in the blood[12]. Our results revealed that UW does not offer any protection against endothelial cell injury. In comparison to livers preserved in UW solution, livers preserved in IGL-1 released markedly less CK-BB after 2 h of reperfusion. Thus, IGL-1 seems to better protect non-parenchymal cells than UW.
During the last few years, several preservation solutions have been developed and studies comparing these solutions to UW have been carried out. Histidine-tryptophan-ketoglutarate (HTK) preservation solution, designed as a cardioplegia liquid, is now used routinely by many centers of abdominal organs preservation. Recent data suggested HTK to be an effective preservation solution of livers[22]. Interestingly, HTK offered a powerful ability to preserve endothelial structure and function during warm ischemia[23].
During storage, the preservation solution is in direct contact with the vascular endothelial bed. Injured endothelial cells are potent producers of cytokines and adhesion molecules that directly facilitate graft invasion by humoral and cellular components of inflammation[24]. These mediators increase damage caused by ischemia or reperfusion and promote acute rejection and chronic rejection. It is reported that HES in UW enhances red blood cell aggregation[4], leading to microvessel occlusive events and inflammation. In contrast, it is described that PEG in UW reduces the inflammatory injury due to cold ischemia-reperfusion in an autotransplanted pig kidney model[25]. Our histological results showed no significant difference for inflammation between the two preserved groups.
In conclusion, data reported here indicate that IGL-1 efficiently preserves parenchymal and non-parenchymal cells associated with orthotopic liver transplantation. Despite the fact that the UW solution is the most commonly used cold storage liquid, HES and K+ limit its performances. IGL-1 (a PEG high-Na UW solution) could represent a valid alternative in organ preservation.
We thank M. Rassas for carefully reading the manuscript and A. Varennes for technical assistance.
S- Editor Wang J L- Editor Kumar M E- Editor Bai SH
| 1. | Yu YY, Ji J, Zhou GW, Shen BY, Chen H, Yan JQ, Peng CH, Li HW. Liver biopsy in evaluation of complications following liver transplantation. World J Gastroenterol. 2004;10:1678-1681. [PubMed] |
| 2. | Bessems M, Doorschodt BM, van Vliet AK, van Gulik TM. Preservation of rat livers by cold storage: a comparison between the University of Wisconsin solution and Hypothermosol. Ann Transplant. 2004;9:35-37. [PubMed] |
| 3. | Cheng Y, Liu YF, Cheng DH, Li BF, Zhao N. Evaluation of CMU-1 preservation solutions using an isolated perfused rat liver model. World J Gastroenterol. 2005;11:2522-2525. [PubMed] |
| 4. | Morariu AM, Vd Plaats A, V Oeveren W, 'T Hart NA, Leuvenink HG, Graaff R, Ploeg RJ, Rakhorst G. Hyperaggregating effect of hydroxyethyl starch components and University of Wisconsin solution on human red blood cells: a risk of impaired graft perfusion in organ procurement. Transplantation. 2003;76:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | van der Plaats A, 't Hart NA, Morariu AM, Verkerke GJ, Leuvenink HG, Ploeg RJ, Rakhorst G. Effect of University of Wisconsin organ-preservation solution on haemorheology. Transpl Int. 2004;17:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Ben Abdennebi H, Steghens JP, Margonari J, Ramella-Virieux S, Barbieux A, Boillot O. High-Na+ low-K+ UW cold storage solution reduces reperfusion injuries of the rat liver graft. Transpl Int. 1998;11:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Hauet T, Han Z, Doucet C, Ramella-Virieux S, Hadj Aïssa A, Carretier M, Papadopoulos V. A modified University of Wisconsin preservation solution with high-NA+ low-K+ content reduces reperfusion injury of the pig kidney graft. Transplantation. 2003;76:18-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Ben Abdennebi H, Steghens JP, Hadj-Aïssa A, Barbieux A, Ramella-Virieux S, Gharib C, Boillot O. A preservation solution with polyethylene glycol and calcium: a possible multiorgan liquid. Transpl Int. 2002;15:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Badet L, Ben Abdennebi H, Petruzzo P, McGregor B, Espa M, Hadj-Aissa A, Ramella-Virieux S, Steghens JP, Portoghese F, Martin X. Effect of IGL-1, a new preservation solution, on kidney grafts (a pre-clinical study). Transpl Int. 2005;17:815-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Badet L, Petruzzo P, Lefrançois N, McGregor B, Espa M, Berthillot C, Danjou F, Contu P, Aissa AH, Virieux SR. Kidney preservation with IGL-1 solution: a preliminary report. Transplant Proc. 2005;37:308-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Oike F, Uryuhara K, Otsuka M, Dehoux JP, Otte JB, Lerut J, Gianello P. Simplified technique of orthotopic liver transplantation in pigs. Transplantation. 2001;71:328-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Vaubourdolle M, Chazouilleres O, Poupon R, Ballet F, Braunwald J, Legendre C, Baudin B, Kirn A, Giboudeau J. Creatine kinase-BB: a marker of liver sinusoidal damage in ischemia-reperfusion. Hepatology. 1993;17:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Tajra LC, Virieux SR, Abdennebi HB, Margonari J, Hadj-Aissa A, Steghens P, Barbieux A, Pozet N, Martin X. Improved function of rat kidney preserved in high sodium University of Wisconsin solution. Transplant Proc. 1996;28:2905-2907. [PubMed] |
| 14. | Ramella SG, Hadj-Aïssa A, Barbieux A, Steghens JP, Colpart JJ, Zech P, Pozet N. Evaluation of a high sodium-low potassium cold-storage solution by the isolated perfused rat kidney technique. Nephrol Dial Transplant. 1995;10:842-846. [PubMed] |
| 15. | Audet M, Alexandre E, Mustun A, David P, Chenard-Neu MP, Tiollier J, Jaeck D, Cinqualbre J, Wolf P, Boudjema K. Comparative evaluation of Celsior solution versus Viaspan in a pig liver transplantation model. Transplantation. 2001;71:1731-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Devlin J, Dunne JB, Sherwood RA, Chambers SM, Tan KC, Peters TJ, Williams R. Relationship between early liver graft viability and enzyme activities in effluent preservation solution. Transplantation. 1995;60:627-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Neil DA, Hubscher SG. Are parenchymal changes in early post-transplant biopsies related to preservation-reperfusion injury or rejection. Transplantation. 2001;71:1566-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Kerkweg U, Jacob M, De Groot H, Mannherz HG, Rauen U. Cold-induced apoptosis of rat liver endothelial cells: contribution of mitochondrial alterations. Transplantation. 2003;76:501-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Straatsburg IH, Abrahamse SL, Song SW, Hartman RJ, Van Gulik TM. Evaluation of rat liver apoptotic and necrotic cell death after cold storage using UW, HTK, and Celsior. Transplantation. 2002;74:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Natori S, Selzner M, Valentino KL, Fritz LC, Srinivasan A, Clavien PA, Gores GJ. Apoptosis of sinusoidal endothelial cells occurs during liver preservation injury by a caspase-dependent mechanism. Transplantation. 1999;68:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 176] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Doeppner TR, Grune T, de Groot H, Rauen U. Cold-induced apoptosis of rat liver endothelial cells: involvement of the proteasome. Transplantation. 2003;75:1946-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Mangus RS, Tector AJ, Agarwal A, Vianna R, Murdock P, Fridell JA. Comparison of histidine-tryptophan-ketoglutarate solution (HTK) and University of Wisconsin solution (UW) in adult liver transplantation. Liver Transpl. 2006;12:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Wilson CH, Stansby G, Haswell M, Cunningham AC, Talbot D. Evaluation of eight preservation solutions for endothelial in situ preservation. Transplantation. 2004;78:1008-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Pearson MJ, Lipowsky HH. Influence of erythrocyte aggregation on leukocyte margination in postcapillary venules of rat mesentery. Am J Physiol Heart Circ Physiol. 2000;279:H1460-H1471. [PubMed] |
| 25. | Hauet T, Goujon JM, Baumert H, Petit I, Carretier M, Eugene M, Vandewalle A. Polyethylene glycol reduces the inflammatory injury due to cold ischemia/reperfusion in autotransplanted pig kidneys. Kidney Int. 2002;62:654-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |