Published online Aug 28, 2006. doi: 10.3748/wjg.v12.i32.5191
Revised: January 8, 2006
Accepted: January 14, 2006
Published online: August 28, 2006
AIM: To estimate the serum α1-antichymotrypsin (ACT), α1-acid glycoprotein (AGP) and transferrin (Tf) concentrations and to evaluate the microheterogeneity of these acute phase proteins in patients with ulcerative colitis.
METHODS: Twenty-seven patients with ulcerative colitis (UC) and 17 healthy control subjects were studied. The patients were categorised as severe (n = 9), moderate (n = 10) and mild groups (n = 8) using Truelove and Witts’ classification of ulcerative colitis. Microheterogeneity of ACT, AGP and Tf was analysed by crossed immunoaffinity electrophoresis (CIAE) with concanavalin A. In all serum samples standard electrophoresis of serum proteins was performed, iron (Fe) concentration, total iron binding capacity (TIBC) and C-reactive protein (CRP) were also measured.
RESULTS: Our patients suffering from ulcerative colitis had significantly higher serum ACT and AGP concentrations and lower serum transferrin concentration in comparison to healthy subjects. Changes in concentrations of acute phase proteins were dependent on the activity of the inflammatory process. The glycosylation patterns of transferrin were related to the inflammation status. We also observed the correlation between ACT and AGP concentrations, patterns of transferrin glycosylation and changes in standard protein electrophoresis or blood cell count.
CONCLUSION: The glycosylation patterns of transferrin obtained from patients suffering from ulcerative colitis are highly branched and sialylated compared with those obtained from healthy subjects. In contrast, the glycosylation patterns of transferrin do not differ according to the activity index of ulcerative colitis. The microheterogeneity patterns of AGP and ACT are similar in ulcerative colitis patients and healthy subjects.
- Citation: Grzymisławski M, Derc K, Sobieska M, Wiktorowicz K. Microheterogeneity of acute phase proteins in patients with ulcerative colitis. World J Gastroenterol 2006; 12(32): 5191-5195
- URL: https://www.wjgnet.com/1007-9327/full/v12/i32/5191.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i32.5191
In ulcerative colitis, a chronic inflammatory disease of unknown etiology, proinflammatory cytokines, such as tumor necrosis factor (TNF), interleukin-1 (IL-1), IL-6 and IL-2, play a major pathophysiological role[1-5]. Acute phase response is a systemic answer to mechanical injuries, infections as well as an unspecific inflammation. Acute phase proteins (APP) play an important role in this non-specific immune reaction. These proteins are produced by the liver in answer to cytokine mediation. The concentrations of many APP have been studied in ulcerative colitis patients[6-8]. Measurement of APP serum concentrations offers a valuable means of assessing the intensity of inflammatory bowel disease. Unsurprisingly, various studies have shown the correlation between these inflammatory mediators and acute phase proteins, such as C-reactive protein (CRP), α1-acid glycoprotein (AGP), α1-antichymotrypsin (ACT) and serum amyloid A (SAA)[9-11]. A acute phase proteins and clinical signs are also of a high value for the treatment and remission of ulcerative colitis. However, the etiological and clinical importance of these proteins is still not completely revealed.
Except for CRP, albumin and SAA, all acute phase proteins are glycoprotiens. Glycosylation is the primary cause of microheterogeneity in proteins. It is an enzyme-directed and site-specific process. Protein sugar prints are conserved, not random and determined independently from the synthetic rate of protein. There is O-linked glycosylation to the hydroxy oxygen of serine and threonine side chains and N-linked glycosylation to the amide nitrogen of asparagine side chains. The N-glycan chains of α1- AGP, α1- ACT and Tf differ in their branching, showing bi-, tri-, and tetra-antennary structures[12-14]. These serum N-glycoproteins physiologically occur in few variants, called microheterogeneity. During acute phase response both concentrations and percentage of individual variants are changed.
Glycosylation may play a role in cell-cell adhesion. The highly branched and sialylated form of AGP which is the ligand for cell adhesion molecules such as E-selectin and P-selectin, inhibits migration of neutrophils, monocytes and T-cells, and ameliorates complement activity[15]. The asialylated carbohydrate-deficient variant of AGP appears mainly in sera of patients after acute inflammation, infection, burn or other severe tissue damage or necrosis[16]. The immunomodulatory properties of AGP have been shown to depend on its glycosylation. The inhibition of lymphocyte proliferation depends on the branching degree of the glycans of AGP[17]. The sialylated acute phase proteins protect against immune complex-induced injury[18]. An increase of sialyl-variants of APP seems to be a mechanism responsible for feedback inhibition of leucocyte migration to inflamed tissues. The heterogeneity of the glycosylation pattern of AGP that has been found in patients with ulcerative colitis reflects changes in response to inflammation[19].
The changes in patterns of glycosylation of transferrin have been observed in iron deficiency anaemia, rheumatoid arthritis, liver cirrhosis or in physiological status such as pregnancy[20]. The microheterogeneity pattern of Tf shifts under inflammatory conditions towards highly branched glycans. Differences in glycosylation of Tf seem to alter the iron metabolism.
The aim of this study was to estimate the changes in microheterogeneity in glycosylation patterns of α1-AGP, α1-ACT and Tf in sera of patients suffering from ulcerative colitis.
Twenty-seven patients suffering from biopsy-proven ulcerative colitis and 17 healthy control subjects were studied.
The sera were separated by centrifugation after clotting and stored at -70°C. Rocket immunoelectrophoresis was used to determine the total serum concentrations of AGP, ACT, Tf, and CRP.
Microheterogeneity of ACT, AGP and Tf was analysed by crossed immunoaffinity electrophoresis (CIAE) with lectin-concanavalin A. The lectin-concanavalin A was included in the first-dimension gel as the diantennary-specific affinocomponent. Separation of different glycoforms of proteins in sera was done via electrophoresis of these fluids with a concanavalin A-containing (Con A) polyacrylamide slab gel. Proteins lacking glycans of the diantennary type are not retarded by Con A, whereas proteins containing one or more diantennary glycans bind to Con A, and are electrophoretically retarded in the gel. Detection of the separated glycoforms was achieved through electrophoresis in the second dimension, using the polyclonal protein-specific anti-IgG antibody. The resulting precipitation lines were stained, and the relative occurrences of lectin-retarded and lectin-nonretarded glycoforms were calculated from the areas under the curves.
AGP, ACT and Tf in sera were separated into four variants: W 0-3 for AGP, A 1-4 for ACT, T 1-4 for Tf. Furthermore, the following parameters were measured: standard electrophoresis of serum proteins, iron (Fe) concentration, total iron binding capacity (TIBC), CRP, haemoglobin concentration and blood cell count.
Twenty-seven patients suffering from ulcerative colitis were studied (16 females, 11 males). The patients were categorised according to the clinical activity index for ulcerative colitis using Truelove and Witts’ classification of ulcerative colitis (severe n = 9, moderate n = 10 and mild n = 8). There were no significant differences in age, gender or body mass index (BMI) among the three studied groups (mean age 41.8 ± 18 years, mean BIM 21.858 ± 3.58 kg/m2). The nutritional status evaluated by physical characteristics was also comparable in the three groups (Table 1).
| Trulove and Witts’ classification | UC (n = 27) | Severe (n = 9) | Moderate (n = 10) | Mild (n = 8) | P |
| Female (n) | 16 | 4 | 6 | 6 | |
| Male (n) | 11 | 5 | 4 | 2 | |
| Age (yr) | 41.80 ± 18.00 | 40.20 ± 20.20 | 38.00 ± 16.00 | 48.40 ± 18.30 | NS |
| Hemoglobin (g/dL) | 12.01 ± 2.21 | 11.17 ± 1.92 | 11.88 ± 2.14 | 13.11 ± 2.38 | < 0.05 |
| PLT (thsd/mm3) | 352.00 ± 190.62 | 428.78 ± 116.65 | 358.90 ± 235.76 | 257.00 ± 173.18 | < 0.05 |
| Fe (μg/dL) | 73.90 ± 35.60 | 52.33 ± 19.20 | 73.20 ± 26.71 | 99.00 ± 45.19 | < 0.05 |
| WBC (thsd/mm3) | 8.68 ± 3.99 | 10.20 ± 5.61 | 9.03 ± 3.13 | 6.58 ± 1.52 | < 0.05 |
| Total protein (g/dL) | 6.15 ± 0.99 | 5.72 ± 1.20 | 6.52 ± 0.71 | 6.20 ± 0.93 | < 0.05 |
| Albumin (g/dL) | 3.22 ± 0.82 | 2.70 ± 0.78 | 3.63 ± 0.59 | 3.40 ± 0.89 | < 0.05 |
The haemoglobin concentration and platelet count were significantly lower in the severe group than in the mild group (11.17 ± 1.92 g/dL and 13.11 ± 2.38 g/dL, respectively, P < 0.05; and 428.78 ± 116.65 thsd/mm3 and 257 ± 173.18 thsd/mm3, respectively, P < 0.05). The serum iron concentration was significantly lower in the severe group than in the moderate (52.3 ± 19.2 µg/dL and 73.3 ± 26.7 μg/dL, respectively, P < 0.05) and mild groups (52.3 ± 19.2 μg/dL and 99 ± 45.2 μg/dL , respectively, P < 0.05). The white blood cell count was higher in the severe group than in the mild group (10.2 ± 5.61 thsd/mm3 and 6.48 ± 1.6 thsd/mm3, respectively, P < 0.05).
There were also significant differences in total protein concentration and albumin concentration between severe and moderate groups (5.72 ± 1.2 g/dL and 6.52 ± 0.71 g/dL, respectively, P < 0.05; 2.7 ± 0.78 g/dL and 3.63 ± 0.59 g/dL, respectively, P < 0.05).
The CRP concentration was significantly higher in the severe group than in the moderate and mild groups (49 ± 34 mg/L and 5 ± 8.4 mg/L, respectively, P < 0.001; 49 ± 34 mg/L and 15 ± 24.42 mg/L, respectively, P < 0.05).
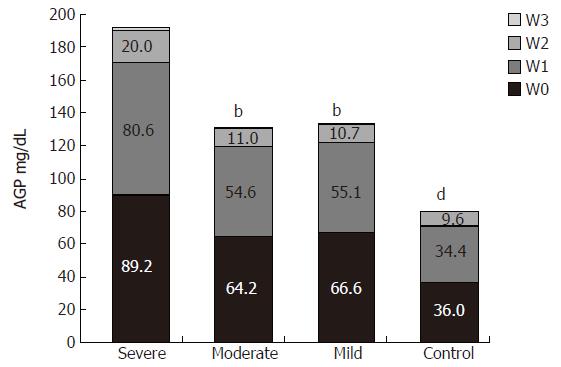
The serum concentration of AGP was significantly higher in all UC patients than in healthy subjects (151.3 ± 65.5 mg/dL and 80 ± 11 mg/dL, respectively, P < 0.001). However, there were also significant differences between the three groups. The highest concentration of AGP was found in the severe group (severe to moderate group: 191.1 ± 77.6 mg/dL and 130.3 ± 40.7 mg/dL, respectively, P = 0.02; severe to mild group: 191.1± 77.6 mg/dL and 132.9 ± 62.4 mg/dL, respectively, P = 0.053) (Figure 1).
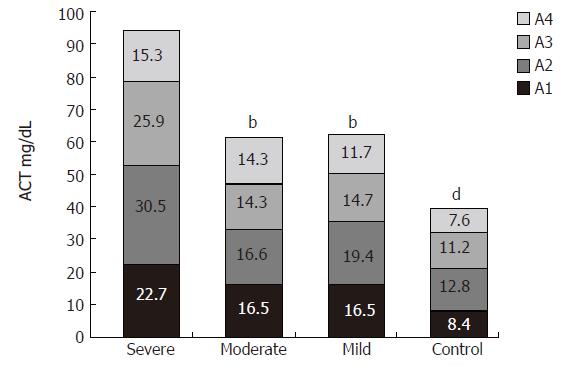
The serum concentration of ACT was also elevated in all studied patients compared to that of the control group (72.8 ± 29.5 mg/dL and 40 ± 5.2 mg/dL, respectively, P < 0.001). Similar to AGP, the ACT concentration was significantly higher in the severe group than in the moderate and mild groups (severe to moderate group: 94.3 ± 36.7 mg/dL and 61.8 ± 18 mg/dL, respectively, P = 0.01; severe to mild group: 94.3 ± 36.7 mg/dL and 62.2 ± 19.2 mg/dL, respectively, P = 0.02) (Figure 2). There were no statistically significant differences in AGP and ACT serum concentrations between the moderate and mild groups.
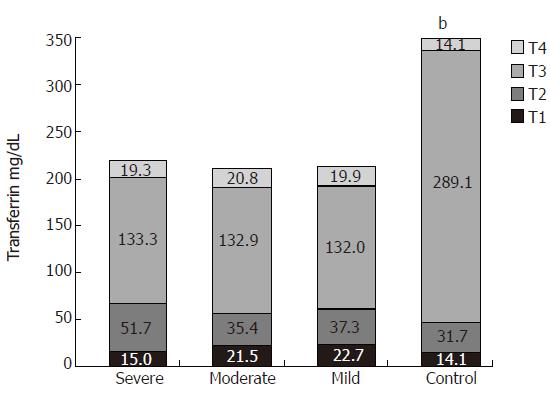
As a negative acute phase protein, serum transferrin concentration was decreased in patients with ulcerative colitis compared to that in the healthy volunteers (237.0 ± 82.3 mg/dL and 352.0 ± 59.1 mg/dL, respectively, P < 0.001). There was no difference in the serum transferrin concentration between the three groups (Figure 3).
The increased concentrations of AGP and ACT were not associated with a large shift in the microheterogeneity patterns.
Interestingly, we noticed a strong positive correlation between the serum AGP concentration, particularly W1 glycoform of AGP and platelet count (r = 0.578, P = 0.0016). The negative correlation was found between the albumin and serum AGP concentrations (r = -0.448, P < 0.05) as well as ACT concentration (r = 0.429, P < 0.05). A particular strong negative relationship occurred between W1 serum fraction of AGP (r = 0.444, P = 0.0296) as well as A2 serum fraction of ACT and albumin concentration (r = 0.578, P = 0.003). There was also a positive correlation between the platelet count and the serum concentration of A2 glycoform of ACT (r = 0.39, P < 0.05).
Decreased serum transferrin concentration in ulcerative colitis patients was accompanied with redirection of glycan synthesis to the highly branched and sialylated glycan. We noticed that the percentage of T2 (bi-, tri- and tetra-antennary) and T1 (tetra- and more antennary) fractions of transferrin were significantly higher in the investigated groups than in healthy subjects, while the percentage of T3 (biantennary) and T4 (asialylated, carbohydrate-deficient) fractions were lower.
We noticed that the correlation between T2 variant of transferrin and platelet count, as well as α2 protein electrophoretic fraction was positive (r = 0.432, P < 0.05 and r = 0.407, P < 0.05). The negative correlation was found between this particular T2 variant and albumin concentration (r = 0.407, P < 0.05).
The positive correlation between the serum concentration of T4 glycoform of transferrin and haemoglobin concentration was observed (r = 0.405, P < 0.05). We also found a negative correlation between T3 glycoform and AGP concentrations, as well as the α1 electrophoretic fraction of proteins (r = 0.48, P < 0.05 and r = 0.475, P < 0.05).
Measurement of acute phase proteins may bring new insights into the mechanisms of inflammatory reactions occurring in ulcerative colitis. In our study, both during remission and exacerbation of ulcerative colitis the glycosylation profiles of AGP and ACT were altered. AGP variant W3 was not present in sera of both healthy individuals and ulcerative colitis patients during remission. However, it appeared in sera of the same patients during exacerbation. This variant is always present in the sera of patients after inflammation, infection, burn or other severe tissue damage. Its presence in the sera of our studied patients was probably caused by the active necrotizing and inflammatory processes in the gut. The high concentration of ACT demonstrated in our patients, is also characteristic for disorders where tissue necrosis occurs (e.g. in burns).
Though the clinical image can confirm ulcerative colitis (UC) is a chronic inflammatory disease, alternations in APP concentrations and microheterogeneity depict rather a domination of “acute inflammatory image” that is probably caused by IL-6. Even in remission of UC the glycosylation profile of the best described AGP does not return to normal but remains altered, with a relative increase of Con A reactive (thus: biantennary) glycoforms. This constant inflammatory stimulation probably contributes to altered Tf glycosylation and, as a consequence to deteriorated iron metabolism and anaemia. In our study, the concentration of transferrin, a negative acute phase protein, was markedly decreased in the acute phase of ulcerative colitis, suggesting that low concentration and especially changes of the glycosylation profile of Tf may be responsible for the impaired iron metabolism in patients suffering from ulcerative colitis.
In sera of healthy individuals, transferrin separates into four variants: T1 weakly reacting with Con A (with mainly triantennary glycans), T2 (with one biantennary glycan and one triantennary glycan), T3 (with two biantennary glycans) and T4 (with probably defective sugars). The variant T3 covers about 80% of the total protein and is the main fraction able to bind to iron, consisting mainly of apotransferrin. Our study showed the alterations of Tf glycosylation in sera of patients suffering from ulcerative colitis. It is well known that existing anaemia in UC patients is not only to the blood loss with a stool, but also due to the chronic inflammatory process. It is possible that the different glycoforms of Tf have a different iron affinity that may affect the iron balance in the organism.
A correlation was shown between the concentration of transferrin T2 variant, bearing partially triantennary glycans and platelet count as well as α-2 protein electrophoretic fraction, whereas a negative correlation was shown between T2 variant and albumin concentration. We are thus able to show that inflammation influences the microheterogeneity of transferrin. The higher intensity of the inflammatory process is combined with deteriorated iron transport ability to transferrin, which may intensify anaemia.
The present study revealed a strong negative correlation between variant T1 of transferrin, α1-acid glycoprotein and α1-antichymotrypsin concentration. A negative correlation was observed between the patient status and iron concentration. Disturbances in the iron balance (low TIBC and iron concentration) reflected a higher amount of variant T1 of transferrin in the sera of UC patients.
In conclusion, glycosylation patterns of transferrin in ulcerative colitis patients shift to the highly branched and sialylated glycans compared with those in healthy subjects. However, the glycosylation patterns of transferrin do not differ according to the activity index of ulcerative colitis. The microheterogeneity patterns of AGP and ACT are similar in ulcerative colitis patients and healthy subjects, though the total concentrations of these acute phase proteins are increased due to the disease.
S- Editor Pan BR L- Editor Wang XL E- Editor Bi L
| 1. | McAlindon ME, Mahida YR. Pro-inflammatory cytokines in inflammatory bowel disease. Aliment Pharmacol Ther. 1996;10 Suppl 2:72-74. [PubMed] |
| 2. | Reimund JM, Wittersheim C, Dumont S, Muller CD, Kenney JS, Baumann R, Poindron P, Duclos B. Increased production of tumour necrosis factor-alpha interleukin-1 beta, and interleukin-6 by morphologically normal intestinal biopsies from patients with Crohn's disease. Gut. 1996;39:684-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 228] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Reimund JM, Wittersheim C, Dumont S, Muller CD, Baumann R, Poindron P, Duclos B. Mucosal inflammatory cytokine production by intestinal biopsies in patients with ulcerative colitis and Crohn's disease. J Clin Immunol. 1996;16:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 202] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Breese EJ, Michie CA, Nicholls SW, Murch SH, Williams CB, Domizio P, Walker-Smith JA, MacDonald TT. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology. 1994;106:1455-1466. [PubMed] |
| 5. | Brynskov J, Nielsen OH, Ahnfelt-Rønne I, Bendtzen K. Cytokines (immunoinflammatory hormones) and their natural regulation in inflammatory bowel disease (Crohn's disease and ulcerative colitis): a review. Dig Dis. 1994;12:290-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Nielsen OH, Vainer B, Madsen SM, Seidelin JB, Heegaard NH. Established and emerging biological activity markers of inflammatory bowel disease. Am J Gastroenterol. 2000;95:359-367. [PubMed] |
| 7. | Niederau C, Backmerhoff F, Schumacher B, Niederau C. Inflammatory mediators and acute phase proteins in patients with Crohn's disease and ulcerative colitis. Hepatogastroenterology. 1997;44:90-107. [PubMed] |
| 8. | Ricci G, D'Ambrosi A, Resca D, Masotti M, Alvisi V. Comparison of serum total sialic acid, C-reactive protein, alpha 1-acid glycoprotein and beta 2-microglobulin in patients with non-malignant bowel diseases. Biomed Pharmacother. 1995;49:259-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Louis E, Belaiche J, van Kemseke C, Franchimont D, de Groote D, Gueenen V, Mary JY. A high serum concentration of interleukin-6 is predictive of relapse in quiescent Crohn's disease. Eur J Gastroenterol Hepatol. 1997;9:939-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 94] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Mazlam MZ, Hodgson HJ. Peripheral blood monocyte cytokine production and acute phase response in inflammatory bowel disease. Gut. 1992;33:773-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Vatay A, Bene L, Kovács A, Prohászka Z, Szalai C, Romics L, Fekete B, Karádi I, Füst G. Relationship between the tumor necrosis factor alpha polymorphism and the serum C-reactive protein levels in inflammatory bowel disease. Immunogenetics. 2003;55:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Agneray J. Glycan microheterogeneity forms of alpha 1-acid glycoprotein (AGP): their identification in biological fluids and variations in their relative proportions in disease states. Prog Clin Biol Res. 1989;300:47-65. [PubMed] |
| 13. | Kalsheker NA. Alpha 1-antichymotrypsin. Int J Biochem Cell Biol. 1996;28:961-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Arndt T. Carbohydrate-deficient transferrin as a marker of chronic alcohol abuse: a critical review of preanalysis, analysis, and interpretation. Clin Chem. 2001;47:13-27. [PubMed] |
| 15. | Lasky LA. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science. 1992;258:964-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 898] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 16. | Fournier T, Medjoubi-N N, Porquet D. Alpha-1-acid glycoprotein. Biochim Biophys Acta. 2000;1482:157-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 712] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 17. | Pos O, Oostendorp RA, van der Stelt ME, Scheper RJ, Van Dijk W. Con A-nonreactive human alpha 1-acid glycoprotein (AGP) is more effective in modulation of lymphocyte proliferation than Con A-reactive AGP serum variants. Inflammation. 1990;14:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Mulligan MS, Lowe JB, Larsen RD, Paulson J, Zheng ZL, DeFrees S, Maemura K, Fukuda M, Ward PA. Protective effects of sialylated oligosaccharides in immune complex-induced acute lung injury. J Exp Med. 1993;178:623-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Rydén I, Skude G, Lundblad A, Påhlsson P. Glycosylation of alpha1-acid glycoprotein in inflammatory disease: analysis by high-pH anion-exchange chromatography and concanavalin A crossed affinity immunoelectrophoresis. Glycoconj J. 1997;14:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | de Jong G, van Noort WL, Feelders RA, de Jeu-Jaspars CM, van Eijk HG. Adaptation of transferrin protein and glycan synthesis. Clin Chim Acta. 1992;212:27-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |