Published online Aug 28, 2006. doi: 10.3748/wjg.v12.i32.5182
Revised: May 18, 2006
Accepted: May 25, 2006
Published online: August 28, 2006
AIM: To investigate the role of wireless capsule endoscopy (WCE) in detection of small bowel (SB) pathology in patients with chronic renal failure (CRF) and obscure bleeding.
METHODS: Consecutive CRF patients with obscure bleeding were prospectively studied. Patients with normal renal function and obscure bleeding, investigated during the same period with WCE, were used for the interpretation of results.
RESULTS: Seventeen CRF patients (11 overt, 6 occult bleeding) and 51 patients (33 overt, 18 occult bleeding) with normal renal function were enrolled in this study. Positive SB findings were detected in 70.6% of CRF patients and in 41.2% of non-CRF patients (P < 0.05). SB angiodysplasia was identified in 47% of CRF patients and in 17.6% of non-CRF patients. Univariate logistic regression revealed CRF as a significant predictive factor for angiodysplasia (P < 0.05). Therapeutic measures were undertaken in 66% of the patients with the positive findings.
CONCLUSION: According to our preliminary results, SB angiodysplasia was found in an increased prevalence among CRF patients with obscure bleeding. WCE is useful in diagnosis of gastrointestinal pathologies and in planning appropriate therapeutic intervention and, therefore, should be included in the work-up of this group of patients.
- Citation: Karagiannis S, Goulas S, Kosmadakis G, Galanis P, Arvanitis D, Boletis J, Georgiou E, Mavrogiannis C. Wireless capsule endoscopy in the investigation of patients with chronic renal failure and obscure gastrointestinal bleeding (preliminary data). World J Gastroenterol 2006; 12(32): 5182-5185
- URL: https://www.wjgnet.com/1007-9327/full/v12/i32/5182.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i32.5182
Unexplained gastrointestinal bleeding (overt or occult) and anemia are common complications of advanced chronic renal failure (CRF)[1,2]. Furthermore, 19% of patients with advanced CRF prior to dialysis and 6% of those on maintenance hemodialysis have hemoccult positive stool[3]. In some cases, upper and lower gastrointestinal tract endoscopic and imaging studies cannot explain these symptoms and findings and bleeding remains a real diagnostic and therapeutic challenge.
Until recently, small bowel (SB) investigation with the available endoscopic and imaging studies was incomplete. Consequently, there are no studies giving information on the pathology of the entire SB in CRF patients, except for two autopsy studies[4,5]. In view of the scarcity of information, we conducted this study and used wireless capsule endoscopy (WCE) to evaluate SB pathology in patients with advanced CRF and obscure bleeding. This novel method is well tolerated and allows complete visual investigation of the SB[6,7]. Moreover, the diagnostic yield of WCE, especially in obscure gastrointestinal bleeding, is significantly higher to that of any other methods, including push enteroscopy, small bowel radiography, computed tomography, magnetic resonance imaging and angiography[8]. To the best of our knowledge, this is the first endoscopic study to explore the entire SB in CRF patients with obscure gastrointestinal bleeding.
This prospective ongoing study included consecutive patients referred to our institution with advanced CRF needing SB investigation for obscure bleeding. Advanced CRF was defined as creatinine clearance of less than 30 mL/min. Renal transplant recipients with stable renal function and serum creatinine concentration ≥ 2 mg/dL for at least a 6-mo period were eligible to enter the study as well.
Age- and sex-matched patients with normal renal function and obscure gastrointestinal bleeding, investigated with WCE during the same period, were used for the interpretation of CRF patients’ findings.
Obscure gastrointestinal bleeding was defined according to the American Gastroenterological Association position statement[9]. Patients with obscure bleeding had been initially investigated with esophagogastroduodenoscopy and colonoscopy in other institutions and, if negative, were subsequently referred for WCE.
Generally accepted contraindications for the WCE procedure are described elsewhere[10]. Written informed consent was obtained from all patients. Patients’ clinical characteristics, including sex, age, total duration of CRF (pre-treatment plus post treatment period), NSAID or aspirin use, comorbid diseases (practically any serious diseases, e.g. cardiovascular, collagen diseases, endocrinopathies, portal hypertension), need for previous transfusions as well as the etiology of renal failure were recorded. Biochemical tests of renal function (serum creatinine and urea) and hemoglobin level were obtained as well.
M2A capsule (Given Imaging, Yoqneam, Israel) was used in this study. Patients’ preparation and WCE procedure were carried out following the generally recommended guidelines[11]. Hemodialysis patients were not given the two-liter solution of polyethylene glycol as preparation, due to restriction of fluid intake; instead, they were advised to abstain from solid food, on the day before the procedure.
A single gastroenterologist (experienced in gastrointestinal endoscopy and WCE) initially screened all videos and selected images of potential abnormalities. Then, two gastroenterologists (also experienced in interpreting WCE findings) independently reviewed the selected images to confirm the accuracy of diagnosis. All videos were extensively discussed and findings identified by both reviewers were considered as definitive and included in the report. The procedure was defined as incomplete if the capsule failed to pass into the cecum during an eight and a half-hour period of the examination. The diagnostic yield of WCE was calculated for both CRF and non-CRF patients. Only lesions with a high potential for bleeding, as defined by Saurin et al[12], were considered.
The Statistical Package for Social Sciences (SPSS) program version 10.0 (Chicago, Illinois) was used for statistical analysis. Continuous data with normal distribution were expressed as mean ± SE, while those without as median (range). Differences between groups were evaluated using χ2 test for qualitative variables and Student’s t test for quantitative variables following a normal distribution or the Mann-Whitney U-test for those who failed the normality test. A P value of less than 0.05 was considered statistically significant. Logistic regression analysis was used to find predictive variables of findings, which were identified in terms of odds ratio (OR) with 95% confidence intervals (95% CI).
A total of 17 CRF patients fulfilled the inclusion criteria (7 predialysis patients, 4 on maintenance hemodialysis and 6 renal transplant recipients). CRF etiology and duration as well as biochemical parameters are shown in Table 1. Duration of dialysis ranged between 20 to 60 mo (mean ± SE: 38.7 ± 3.8) and the treatment schedule consisted of 4 h hemodialysis procedures three times weekly. Post-transplantation period ranged between 16 to 93 mo (mean ± SE: 53.2 ± 12.5). Immunosuppressive treatment in transplant recipients consisted of a combination of mycophenolate mofetil (MMF), cyclosporine and prednisone (3/6 patients), MMF, tacrolimus and prednisone (2/6) and MMF, sirolimus and prednisone (1/6). Two out of 17 CRF patients were taking low-dose aspirin and 1 non-steroidal anti-inflammatory drug (NSAID).
| No. of patients | 17 |
| Etiology of renal failure, n (%) | |
| Nephrosclerosis | 4 (23.5) |
| Glomerulonephritis | 3 (17.6) |
| Chronic pyelonephritis | 1 (5.9) |
| Diabetic nephropathy | 1 (5.9) |
| Other etiologies | 3 (17.6) |
| Unknown | 5 (29.4) |
| Duration of CRF ( mean ± SE), mo | 139.9 ± 13.0 |
| Median serum creatinine (range), mg/dL | 3.2 (2.0 - 9.4) |
| Serum urea (mean ± SE), mg/dL | 133.9 ± 11.0 |
The group of non-CRF patients consisted of 51 patients with obscure bleeding. Table 2 shows the demographic and clinical characteristics of CRF and non-CRF patients. The two groups were found comparable regarding indications for WCE (overt, occult bleeding), NSAID or aspirin use, comorbid diseases, hemoglobin level and need for previous transfusions.
| CRF patients | Non-CRFpatients | P | |
| No. of patients | 17 | 51 | |
| Male/female | 12/5 | 36/15 | NS |
| Age (mean ± SE), yr | 57.1 ± 2.5 | 57.2 ± 2.1 | NS |
| Indication of WCE (overt/occult bleeding) | 11/6 | 33/18 | NS |
| NSAIDs or aspirin use, n (%) | 3/17 (17.6%) | 14/51 (27.5%) | NS |
| Comorbid diseases, n (%) | 10/17 (58.8%) | 23/51 (45.1%) | NS |
| Hb (mean ± SE), g/L | 84.3 ± 2.0 | 87.7 ± 1.3 | NS |
| Need for previous transfusions, n (%) | 6/17 (35.3%) | 28/51 (54.9%) | NS |
CRF patients completed the procedure uneventfully. One case of capsule retention was observed in a non-CRF patient with an adenocarcinoma of the mid ileum. In 3 (17.6%) CRF and 10 (19.6%) non-CRF patients, the capsule did not reach the colon, and, therefore, the entire SB was not imaged. Causes of failure of the capsule to reach the colon within the recording time in CRF patients were: slow gastric passage (1 case), presence of food impairing the capsule progression (1 case), and no clear reason (1 case). Gastric emptying time in CRF patients ranged from 5 to 288 min (median: 20) and SB transit time from 99 to 393 min (median: 275).
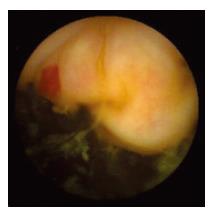
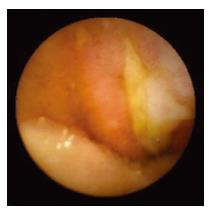
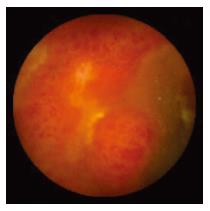
WCE findings of SB in both CRF and non-CRF patients are listed in Table 3. In CRF patients, the most frequent type of SB lesion identified was angiodysplasia (8/17, 47%; two actively bleeding) (Figure 1). Patchy mucosal redness and a big jejunal ulcer (Figure 2) were revealed in 2 patients, 1 of which had a recent history of NSAIDs use. Ulceration, cobblestoning, stricture and a pattern of discontinuous involvement of the mid ileum, suggestive of Crohn’s disease, were found in 1 patient. Finally, in 1 patient with erythema, edema, submucosal bleeding, erosions and non-specific ulcers of the distal ileum, caecum and ascending colon (Figure 3), tissue diagnosis of CMV enterocolitis was subsequently made by means of ileocolonoscopy and biopsies. Preceded colonoscopy in another institution, ten days earlier, had missed the lesions. Consequently, the diagnostic yield of WCE in CRF patients with obscure bleeding was significantly higher (70.6%, 12/17) compared with the non-CRF patients (41.2%, 21/51) (χ2 = 4.42, P < 0.05).
| SB findings | CRF patientsn = 17 (%) | Non-CRF patientsn = 51 (%) |
| Angiodysplasia | 8 (47.0) | 9 (17.6) |
| Single ulcer | 2 (11.8) | 3 (5.9) |
| Ulceration with cobblestoning and stricture | 1 (5.9) | 3 (5.9) |
| Non-specific ulcers, erosions, submucosal bleeding, erythema and edema | 1 (5.9) | - |
| Fresh blood without an obvious explanation | - | 2 (3.9) |
| Tumor | - | 2 (3.9) |
| Multiple erosions | - | 2 (3.9) |
| No findings | 5 (29.4) | 30 (58.8) |
Angiodysplasia was the most frequent finding in the SB of CRF patients. A single lesion was found in 5 patients, 2 lesions in 2 and 3 in 1. Angiodysplasias were located in jejunum (5 patients), ileum (2 patients) and both (1 patient).
We assessed presence of CRF, age, sex, comorbid diseases, hemoglobin level at the time of the procedure and need for previous transfusions as potentially relevant independent variables for angiodysplasia in both CRF and non-CRF patients. Univariate logistic regression revealed only CRF as a significant predictive factor for angiodysplasia (OR = 4.1, 95% CI = 1.3-13.7, P < 0.05). Moreover, we evaluated sex, age, duration of CRF and comorbid diseases as potentially relevant independent variables for SB angiodysplasias in CRF patients. SB angiodysplasias seem to be associated only with the duration of CRF (OR = 1.03, 95% CI = 0.99-1.06, P < 0.1).
In view of the WCE findings, 4/8 patients with angiodysplastic lesions within the reach of push enteroscopy were referred for endoscopic treatment with argon plasma coagulation and 1 patient with 3 angiodysplasias received estrogen therapy. Pharmacotherapy was provided in the patient with Crohn's disease as well as in the patient with CMV enterocolitis, and discontinuation of NSAIDs in the patient with the big jejunal ulcer was undertaken. Iron supplementation was decided to be the only measure in the remaining 3 cases with angiodysplasias and in the patient with the jejunal ulcer.
Gastrointestinal bleeding is a common complication of advanced CRF[1,2]. Although there have been many studies concerning implication of upper gastrointestinal tract and colon, data concerning SB are limited. WCE is a new method for the investigation of SB pathology, but already has an established role in cases of obscure gastrointestinal bleeding[8]. We conducted this study in order to investigate SB pathology in CRF patients with obscure gastrointestinal bleeding and assess the diagnostic and therapeutic yield of the method in this group of patients. To our knowledge, there is only one case report on this issue[13].
According to the preliminary results of our ongoing study, angiodysplasia was recognized as a sole causative lesion of obscure bleeding in a markedly higher percentage, 47% in CRF patients vs 17.6% in non-CRF patients. A high prevalence (66.7%) of SB angiodysplasia among 6 CRF patients has also been reported in a recent study on the impact of WCE in obscure gastrointestinal bleeding[14]. In our study, logistic regression analysis showed that CRF was a significant predictive factor for SB angiodysplasia (OR = 4.1, 95% CI = 1.3-13.7, P < 0.05). Furthermore, our data suggested that the prevalence of angiodysplasia seemed to be inversely related with the duration of CRF, although this finding was statistically insignificant (OR = 1.03, 95% CI = 0.99-1.06, P < 0.1). Previous reports on the prevalence of angiodysplasia in CRF patients, as well as its association with patient’s age and duration of the disease are conflicting[15-18]. However, all those studies used data obtained by conventional upper gastrointestinal endoscopy.
The diagnostic yield of WCE in CRF patients with obscure bleeding was significantly higher (70.6%) than that in non-CRF patients (χ2 = 4.42, P < 0.05), which is in agreement with previous data[19-21]. Given that WCE has already become the method of choice in the investigation of patients with obscure bleeding[8], its value in the subgroup of CRF patients is even more crucial.
WCE findings led to plan a specific intervention in 8/12 patients with positive findings. Therapeutic measures included push enteroscopy with coagulation of angiodysplasias in 4 patients, pharmacotherapy in 3 and discontinuation of medication in another 1.
In conclusion, according to the preliminary results of our ongoing study, SB angiodysplasia has an increased prevalence among CRF patients with obscure bleeding. WCE is useful in diagnosis of gastrointestinal pathologies and in planning appropriate therapeutic intervention and, therefore, should be included in the work-up of this group of patients.
S- Editor Wang J L- Editor Kumar M E- Editor Liu WF
| 1. | Etemad B. Gastrointestinal complications of renal failure. Gastroenterol Clin North Am. 1998;27:875-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Kang JY. The gastrointestinal tract in uremia. Dig Dis Sci. 1993;38:257-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 98] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Akmal M, Sawelson S, Karubian F, Gadallah M. The prevalence and significance of occult blood loss in patients with predialysis advanced chronic renal failure (CRF), or receiving dialytic therapy. Clin Nephrol. 1994;42:198-202. [PubMed] |
| 4. | Vaziri ND, Dure-Smith B, Miller R, Mirahmadi MK. Pathology of gastrointestinal tract in chronic hemodialysis patients: an autopsy study of 78 cases. Am J Gastroenterol. 1985;80:608-611. [PubMed] |
| 5. | Chachati A, Godon JP. Effect of haemodialysis on upper gastrointestinal tract pathology in patients with chronic renal failure. Nephrol Dial Transplant. 1987;1:233-237. [PubMed] |
| 6. | Meron GD. The development of the swallowable video capsule (M2A). Gastrointest Endosc. 2000;52:817-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 127] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405:417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1994] [Cited by in RCA: 1384] [Article Influence: 55.4] [Reference Citation Analysis (1)] |
| 8. | Triester SL, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100:2407-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 419] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 9. | American Gastroenterological Association medical position statement: evaluation and management of occult and obscure gastrointestinal bleeding. Gastroenterology. 2000;118:197-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 108] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Eliakim R. Wireless capsule video endoscopy: three years of experience. World J Gastroenterol. 2004;10:1238-1239. [PubMed] |
| 11. | Gay G, Delvaux M, Rey JF. The role of video capsule endoscopy in the diagnosis of digestive diseases: a review of current possibilities. Endoscopy. 2004;36:913-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Saurin JC, Delvaux M, Gaudin JL, Fassler I, Villarejo J, Vahedi K, Bitoun A, Canard JM, Souquet JC, Ponchon T. Diagnostic value of endoscopic capsule in patients with obscure digestive bleeding: blinded comparison with video push-enteroscopy. Endoscopy. 2003;35:576-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 315] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 13. | Brümmer U, Cappelli P, Laterza F, Di Liberato L, Sirolli V, Milano A, Mastrippolito S, D'Arezzo M, Muscianese P, Amoroso L. Wireless capsule endoscopy in the diagnostic of small intestine angiodysplasia in chronic uremic patient. Minerva Urol Nefrol. 2005;57:61-69. [PubMed] |
| 14. | Viazis N, Papaxoinis K, Theodoropoulos I, Sgouros S, Vlachogiannakos J, Pipis P, Markoglou C, Avgerinos A. Impact of capsule endoscopy in obscure small-bowel bleeding: defining strict diagnostic criteria for a favorable outcome. Gastrointest Endosc. 2005;62:717-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Chalasani N, Cotsonis G, Wilcox CM. Upper gastrointestinal bleeding in patients with chronic renal failure: role of vascular ectasia. Am J Gastroenterol. 1996;91:2329-2332. [PubMed] |
| 16. | Zuckerman GR, Cornette GL, Clouse RE, Harter HR. Upper gastrointestinal bleeding in patients with chronic renal failure. Ann Intern Med. 1985;102:588-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 119] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Ala-Kaila K. Upper gastrointestinal findings in chronic renal failure. Scand J Gastroenterol. 1987;22:372-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Sotoudehmanesh R, Ali Asgari A, Ansari R, Nouraie M. Endoscopic findings in end-stage renal disease. Endoscopy. 2003;35:502-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Delvaux M, Fassler I, Gay G. Clinical usefulness of the endoscopic video capsule as the initial intestinal investigation in patients with obscure digestive bleeding: validation of a diagnostic strategy based on the patient outcome after 12 months. Endoscopy. 2004;36:1067-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Rastogi A, Schoen RE, Slivka A. Diagnostic yield and clinical outcomes of capsule endoscopy. Gastrointest Endosc. 2004;60:959-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | De Leusse A, Landi B, Edery J, Burtin P, Lecomte T, Seksik P, Bloch F, Jian R, Cellier C. Video capsule endoscopy for investigation of obscure gastrointestinal bleeding: feasibility, results, and interobserver agreement. Endoscopy. 2005;37:617-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |