Published online Aug 28, 2006. doi: 10.3748/wjg.v12.i32.5153
Revised: April 15, 2006
Accepted: April 21, 2006
Published online: August 28, 2006
AIM: To study the effect of oxymatrine-baicalin combination (OB) against HBV replication in 2.2.15 cells and α smooth muscle actin (α SMA) expression, type I, collagen synthesis in HSC-T6 cells.
METHODS: The 2.2.15 cells and HSC-T6 cells were cultured and treated respectively. HBsAg and HBeAg in the culture supernatants were detected by ELISA and HBV DNA levels were determined by fluorescence quantitative PCR. Total RNA was extracted from HSC-T6 cells and reverse transcribed into cDNA. The cDNAs were amplified by PCR and the quantities were expressed in proportion to β actin. The total cellular proteins extracted from HSC-T6 cells were separated by electrophoresis. Resolved proteins were electrophoretically transferred to nitrocellulose membrane. Protein bands were revealed and the quantities were corrected by β actin.
RESULTS: In the 2.2.15 cell culture system, the inhibitory rate against secretion of HBsAg and HBeAg in the OB group was significantly stronger than that in the oxymatrine group (HBsAg, P = 0.043; HBeAg, P = 0.026; respectively); HBV DNA level in the OB group was significantly lower than that in the oxymatrine group (P = 0.041). In HSC-T6 cells the mRNA and protein expression levels of α SMA in the OB group were significantly lower as compared with those in the oxymatrine group (mRNA, P = 0.013; protein, P = 0.042; respectively); The mRNA and protein expression levels of type I collagen in the OB group were significantly lower as compared with those in the oxymatrine group (mRNA, P < 0.01; protein, P < 0.01; respectively).
CONCLUSION: OB combination has a better effect against HBV replication in 2.2.15 cells and is more effective against α SMA expression and typeI collagen synthesis in HSC-T6 cells than oxymatrine in vitro.
- Citation: Cheng Y, Ping J, Xu HD, Fu HJ, Zhou ZH. Synergistic effect of a novel oxymatrine-baicalin combination against hepatitis B virus replication, α smooth muscle actin expression and type I collagen synthesis in vitro. World J Gastroenterol 2006; 12(32): 5153-5159
- URL: https://www.wjgnet.com/1007-9327/full/v12/i32/5153.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i32.5153
The human hepatitis B virus (HBV) belongs to the family of small DNA hepadnaviruses. HBV infection is a major cause of chronic hepatitis, hepatic fibrosis, liver cirrhosis, and hepatocellular carcinoma and results in one million deaths annually[1]. In China there are about thirty million patients suffering from chronic hepatitis B. Hepatic fibrosis is a kind of compensating and healing response to liver injuries. And hepatic fibrogenesis has been known to be a gradual and dynamic process associated with the continuous deposition and resorption of connective tissues and collagens[2,3]. During the hepatic fibrogenesis process, hepatic stellate cells (HSC, formerly termed as lipocytes, Ito cells or fat-storing cells) play a central role based on their ability to undergo activation following liver injury of any cause. HSC has been recognized to be responsible for most of the excess extracellular matrix (ECM) observed in chronic hepatic fibrosis[4].
For a few decades, interferon α (IFN α) has been the only approved therapy for chronic HBV infection around the world. But its efficacy is not satisfactory and associated with some adverse reactions such as influenza-like syndrome, leukocyte and platelet decrease[5]. Recently, lamivudine has been the first nucleotide analog approved for treating chronic HBV infection in many regions of the world; but its efficacy is just similar to IFN α and prolonged administration is associated with drug resistance and virus variation, which could lead to severe consequences including liver failure[6] and even death[7]. Up to now, the treatment of HBV is still a difficult problem. Therefore, it is necessary to develop novel drugs and treatment methods.
Oxymatrine is a kind of alkaloid extraction derived from a Chinese herb Sophora flavescens Ait[2,3]. It has been widely used for treating viral hepatitis B and C and hepatic fibrosis in recent years in China[8,9]. But just like other anti-HBV drugs, how to improve its efficacy against HBV and hepatic fibrosis is still an urgent challenge in clinical practice. On the basis of our previous findings[10], in the present study, the 2.2.15 cells[11], the hepatoblastoma cell line HepG2 transfected with cloned hepatitis B virus DNA, and the rat HSC-T6 cells[12], an immortalized rat hepatic stellate cell line, were cultured respectively, and the effects of a novel oxymatrine-baicalin combination[10] (OB) against HBV replication and α smooth muscle actin (α SMA) expression, type I collagen synthesis in vitro were evaluated.
Oxymatrine, and OB combination were prepared and provided by Shanghai Kairuisi Biotech Co. Ltd. according to our applied China National Invention Patent[13]. Dulbecco’s modified Eagle’s medium (DMEM) and modified Eagle’s medium (MEM) culture media were the products of Gibco BRL. Fetal bovine sera (FBS) were purchased from Hyclone (Logan, Utah, USA). G418 was purchased from Shanghai Jiebeisi Gene-Tech Co. Ltd. L-glutamine and EDTA were supplied by Shanghai Shisheng Biotech Co. Ltd. The ELISA kits for HBsAg (hepatitis B surface antigen) and HBeAg (hepatitis B e antigen) kits were ordered from Huamei Biotech Co. Ltd. The penicillin and streptomycin were the products of Shanghai Xianfeng Pharmacological Co.
The HBV DNA (deoxyribonucleic acid) PCR (polymerase chain reaction)-fluorescence quantitative diagnostic kit was purchased from Shanghai Kehua Bio-engineering Co. Ltd. Trizol reagent was the product of Invitrogen. DNA marker was purchased from Tiangen Co. The cDNA synthesis kit and PCR master mix kit were purchased from Fermentas Co. The specific primers were synthesized by Shanghai Shenggong Co. The rabbit anti-rat type I collagen polyclonal antibody was the product of Merck. The mouse anti-rat α SMA mAb and anti-rat β actin mAb were purchased from Sigma (St. Louis, MO). The DC protein quantification kit was the product of Bio-Rad (Bio-Rad Laboratories, Hercules, CA, USA). Enhanced chemiluminescence reagents were purchased from Pirce. The other reagents routinely used in our laboratory were all of analytical grade.
Cell culture flasks and plates were the products of Corning Inc (Corning, NY, USA). CO2 culture hood and Healforce hood were the products of Heraeus Co. Labsystems Multiskan MS Microplate Reader was made in Finland. XDS-1B invert phase-contrast microscope was purchased from Chongqin Guangdian Co. The LightCycler fluorescence PCR system was the product of Roche Co. The Rotor-Gene RG-3000 PCR machine was the product of Gene Co. The Furi FR-980 image analysis system was provided by Shanghai Furi Co. Western blot instrument was produced by Bio-Rad.
The 2.2.15 cell line was purchased from the Department of Microbiology, Tianjin Medical University, China. The 2.2.15 cells were cultured in complete MEM culture media (supplemented with 100 mL/L FBS, 3.8 g/L L-glutamine, 0.38 g/L G418, 50 ku/L penicillin and streptomycin, pH 7.0) at 37°C in 50 mL/L CO2, 950 mL/L air. When cells were in the logarithmic growth phase, they were trypsinized and seeded. Experiments were performed when cells reached 80% confluence. The cells were supplemented with oxymatrine or OB combination and incubated for an additional 4 d. The working concentration of oxymatrine was 1 g/L and the OB combination contained 1 g/L oxymatrine[10] and a specific concentration of baicalin[13] according to our previous findings. At the above concentrations these drugs have no cytotoxic effect to cells by MTT colorimetric assay[10]. Then the cell culture medium was collected according to the experimental protocol. All experiments were performed in duplicate or triplicate samples.
HSC-T6 cells were kindly provided by Professor Scott Friedman (Liver Center Laboratory, San Francisco General Hospital, USA) and has been stored and passaged routinely in our institute. HSC-T6 cells were seeded in DMEM with 100 mL/L FBS at 37°C, in 50 mL/L CO2, 950 mL/L air. When cells were in the logarithmic growth phase, they were trypsinized and seeded into 60 mm culture plates. Experiments were performed when cells reached 80% confluence. Each plate was supplemented with 2 mL culture media with oxymatrine or OB combination and incubated for an additional 24 h. Then the cells were harvested according to the experimental protocol. All experiments were performed in duplicate or triplicate samples.
The 2.2.15 cells were incubated at a density of 1 × 109/L in 1 L MEM medium containing 100 mL/L FBS. After 24 h incubation, the 2.2.15 cells were treated with oxymatrine or OB combination respectively. Cells were cultured in the presence of drugs for 4 d. Then the supernatants were collected and stored at -20°C until measurement. The HBsAg and HBeAg were detected simultaneously by ELISA kits according to the manufacturer’s instruction, and the inhibitory rates for HBsAg and HBeAg were calculated respectively.
The HBV DNA level in the supernatants post-drug treatment was determined by the HBV DNA PCR-quantitative diagnostic kit using the Roche LightCycler system according to the manufacturer’s protocol. One hundred microliter culture medium was added to 100 μL sample reagent A, and then centrifuged at 13 000 r/min for 10 min. The supernatants were discarded and 25 μL sample reagent B was added. After centrifugation for a few seconds, the mixture was heated at 100°C for 10 min. Then 2 μL of the supernatants after centrifugation at 13 000 r/min for 10 min was mixed with 18 μL PCR reaction reagent, and analyzed at Channel F1 by the LightCycler system.
Total RNA was extracted from HSC-T6 cells by Trizol reagent following the protocols provided by the manufacturer. The integrity of total RNA was confirmed by the denaturing formaldehyde agarose gel electrophoresis. The quantity and purity of RNA were detected by determining absorbance at 260/280 nm using a spectrophotometer. Total RNA was reverse transcribed into complementary DNA (cDNA) using the cDNA synthesis kit. The reverse-transcription (RT) reaction mixtures were amplified by semi-quantitative PCR using specific primers for the target genes and β actin. The primers used are as follows: Type I collagen, forward: 5'-TAC AGC ACG CTT GTG GAT G-3', reverse: 5'-TTG AGT TTG GGT TGT TGG TC-3', target fragment length 259 bp; α SMA, forward: 5'-CCG ACC GAA TGC AGA AGG-3', reverse: 5'-ACA GAG TAT TTG CGC TCC GGA-3',target fragment length 88 bp; β actin, forward: 5'-TGA CGA GGC CCA GAG CAA GA-3', reverse: 5’-ATG GGC ACA GTG TGG GTG AC-3’, target fragment length 330 bp. The 20 μL reaction mixture consisted of the corresponding primers, 1 U of Taq polymerase, 50 μmol/L of each of the four dNTP, 1 × PCR buffer supplemented with 2.5 mmol/L MgCl2. After an initial melting time of 5 min at 94°C, the mixtures were subjected to 35 PCR cycles, consisting of denaturation for 1 min at 94°C, primer annealing for 1 min at 55°C, and primer extension for 1 min at 72°C, with a final extension time of 10 min at 72°C. Quantification of the final products was performed by electrophoresis in 30 g/L agarose gel using the Furi FR-980 image analysis system. The number of mRNA molecules was expressed in proportion to the number of internal control β actin in the same sample as described before[14].
HSC-T6 cell lysates were prepared from 1 × 107 cells by dissolving cell pellets in 100-200 μL lysis buffer (150 mmol/L NaCl, 10 g/L tergitol NP-40, 5 g/L sodium deoxycholate, 0.1 g/L sodium dodecyl sulfate, 50 mmol/L Tris, and a protease inhibitor cocktail, pH7.5). Lysates were centrifuged at 4°C, 12 000 r/min for 15 min, then the supernatants were collected and stored at -70°C until detection. Protein content was analyzed by DC protein assay and at 690 nm wavelength. Sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (0.31 mL 2 mol/L Tris buffer (pH6.8), 20 g/L SDS, 100 mL/L glycerol, 2.4 g/L DTT, 50 g/L β-mercaptoethanol, 0.02 g/L bromophenol blue) was added to the lysates. After mixing the lysates were heated to 100°C for 5 min, and 50 μg sample protein was loaded into each well of 100 g/L SDS-PAGE gel. Resolved proteins were electrophoretically transferred to nitrocellulose membrane and blocked with 50 mL/L non-fatty milk. Then the primary antibodies (mouse anti-rat β actin antibody, 1:5000 dilution; mouse anti-rat α SMA antibody, 1:5000 dilution, rabbit anti-rat type I collagen antibody, 1:200 dilutions) in 50 mL/L non-fatty TTBS solution (1.21 g/L Tris, 9 g/L NaCl, 1 g/L Tween-20, pH7.5) were added respectively. After incubation at 4°C overnight the blots were washed, then membranes were incubated with corresponding horseradish peroxidase-conjugated secondary antibodies for 1 h. Protein bands were revealed by the ECL kit according to the manufacturer’s protocol and the Furi FR-980 image analysis system was used for quantitative analysis of the blots. β actin protein was used as the internal control as described before[15].
All results were expressed as mean ± SD. Comparisons were analyzed by one-way ANOVA using the SPSS 10.0 statistical package. Differences were considered statistically significant if the P < 0.05.
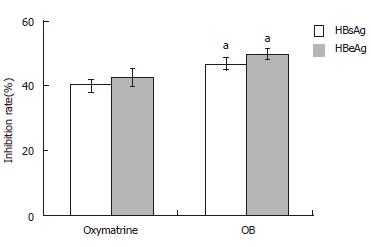
After incubation for 4 d, HBsAg and HBeAg secretion in the culture medium was determined by ELISA. As shown in Figure 1, both OB and oxymatrine had remarkable inhibitory effects on secretion of HBsAg and HBeAg in the 2.2.15 cells, the inhibitory rate in the OB group was significantly stronger than that in oxymatrine group (HBsAg, P = 0.043; HBeAg, P = 0.026; respectively). And the inhibitory rate on HBeAg in both groups exceeded that of HBsAg.
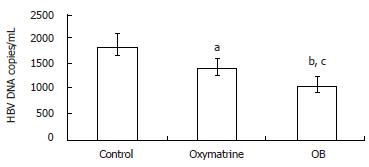
After incubation for four days, the levels of HBV DNA in the culture medium were determined by fluorescence quantitative PCR. As shown in Figure 2, both OB and oxymatrine reduced the HBV DNA level in the supernatant of the 2.2.15 cells, and the HBV DNA level in the OB group was significantly lower than that of the oxymatrine group (P = 0.041), indicating that OB inhibited HBV DNA replication more effectively than oxymatrine did.
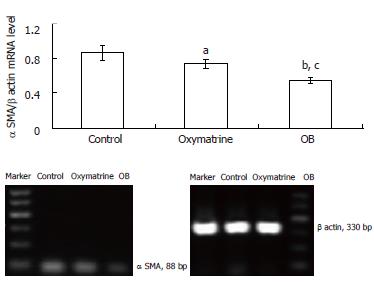
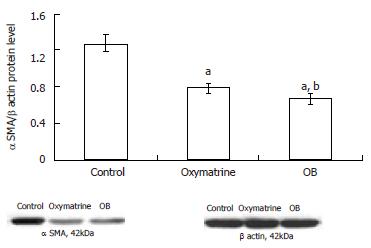
The α SMA mRNA and protein expression levels in HSC-T6 cells were determined by semi-quantitative PCR and Western blot analysis respectively. The β actin mRNA and protein were chosen as internal controls. The results showed that the mRNA and protein expression levels of α SMA in both the oxymatrine and OB groups were significantly reduced compared with the control group (mRNA, P = 0.033 and P = 0.001 vs the control; protein, P < 0.01 and P < 0.01 vs the control; respectively). As shown in Figures 3 and 4, the mRNA and protein expression levels of α SMA in the OB group were significantly lower as compared with those in the oxymatrine group, suggesting that OB reduced the HSC-T6 activation more effectively than oxymatrine (mRNA, P = 0.013; protein P = 0.042; respectively).
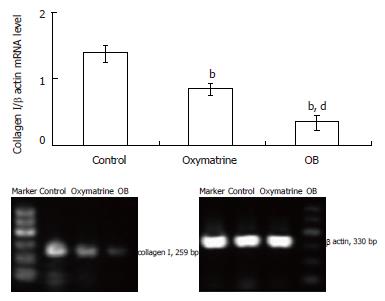
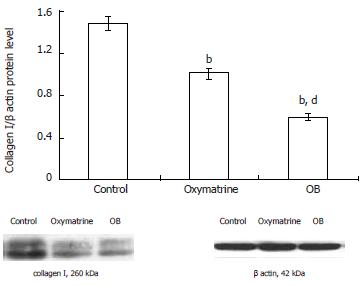
The type I collagen mRNA and protein expression levels were also determined. As shown in Figures 5 and 6, the mRNA and protein expression levels in both the oxymatrine and OB groups were significantly reduced compared with the control group (mRNA, P < 0.01 and P < 0.01; protein, P < 0.01 and P < 0.01; respectively). The results showed that the mRNA and protein expression levels in the OB group were significantly lower as compared with those in the oxymatrine group, suggesting that the OB combination reduced the synthesis of type I collagen in HSC-T6 cells more effectively than oxymatrine not only at mRNA level but also at the protein level (mRNA, P < 0.01; protein, P < 0.01; respectively).
The World Health Organization places HBV infection among the top 10 causes of death worldwide. It is estimated that there are over 400 million carriers of HBV. At least 20% to 30% of HBsAg carriers will die of complications of chronic liver disease, including cirrhosis and liver cancer. The serious consequences of end-stage liver disease and liver cancer occur in 30% of chronic carriers[1,5,16]. Investigation of the expression and replication of the HBV genome has been hampered by the lack of an in vitro tissue culture system until the 2.2.15 cell line was established[11]. The medium of these cells contains not only all of the particles present in the serum of infected individuals but also a number of replicative intermediates that probably represent recircularized, ccc, and single-stranded HBV DNA. Various parameters of the replicative cycle can be quantitated in the 2.2.15 culture system, for example, the secretion of HBsAg or HBeAg and the amount of episomal HBV DNA. At present, the 2.2.15 cell line is still the main in vitro model used for evaluating the anti-HBV effect of drugs[10].
Because HBV infection plays a key role in the development of decompensated cirrhosis and hepatocellular carcinoma, HBV patients with serum aminotransferase twice the upper limit of reference value and HBV DNA positive in the blood were advised to receive antiviral agents treatment[5,8]. Oxymatrine has been used widely in the treatment of chronic liver disease including hepatitis B, hepatitis C and hepatic fibrosis[2,3,7,8]. As for etiological treatment, oxymatrine could effectively treat chronic viral hepatitis and promote the serum markers of HBV in chronic hepatitis B patients to convert to negative and reduce serum level of ALT[8,9]. But its efficacy is similar to IFN α, therefore, it is urgent to improve the treatment efficacy of oxymatrine. The present study showed that the inhibitory rate of OB combination against HBV antigens secretion in the supernatant of the 2.2.15 culture system was significantly stronger than that of oxymatrine. In order to elucidate the influence of OB combination on HBV replication, HBV DNA was determined in the supernatants also and the results indicated that OB combination could suppress virus replication and the effect was superior to oxymatrine treatment alone. The pattern of OB combination against HBV is consistent with those reports on oxymatrine elsewhere[16,17].
The objective of anti-HBV therapy is not only to eradicate the virus but also to prevent the development of hepatic fibrosis. Hepatic fibrosis, a precursor of cirrhosis, is a consequence of severe liver damage that occurs in many patients with chronic liver disease, and involves the abnormal accumulation of ECM[2,3,9,14]. Liver fibrosis represents a major healthcare burden worldwide. During hepatic injury, HSCs become active and undergo profound phenotypic changes[4,18]. HSCs are non-parenchyma cells, located perisinusoidally in the space of Disse. The expression of α SMA is the most important feature of HSC activation. HSC is the primary source of excessive production of ECM. The collagens are the major ECM component of normal and fibrotic livers. In the normal liver the amount of type III collagen is greater than type I collagen, but type I collagen is particularly produced predominantly during fibrogenesis. While the primary HSC cultures are a useful tool for studying hepatic fibrogenesis, their isolation is extremely time-consuming, the yields are modest, and there is considerable preparation-to-preparation variability. The rat HSC-T6 cell line[12] is constructed through transfecting SV40 into rat HSC and its phenotype is activated HSC. HSC-T6 has the stable phenotype and biochemical characters of activated HSC, expressing myogenic and neural crest cytoskeletal filaments, and the cell line has been a useful tool for studying hepatic fibrogenesis and it is also a reliable cell model for investigating antifibrotic drugs[19].
In this study the RT-PCR and Western blot analysis results showed that OB combination had a stronger inhibitory effect against the activation of HSC, and this was proven by the significantly lower levels of α SMA mRNA and protein in the OB group than those in the oxymatrine group. Type I collagen is produced predominantly during hepatic fibrogenesis[4,18]. Inhibiting the secretion of type I collagen not only reduced the ECM component, but also decreased further activation of HSC[19]. Our results showed that the OB combination could not only inhibited the synthesis of type I collagen at the transcription level but also at the translation level, and these inhibitory effects of the OB combination were superior to those of oxymatrine.
In addition to the anti-HBV effect[8,16], it was reported that oxymatrine has an effect against liver fibrosis in vitro[15] and in vivo[2,3,17,21], protects animals from fulminant hepatitis[22] or inflammation[23]. Baicalin, a flavonoid isolated from the root of Scutellaria baicalensis Georgi[10], has been demonstrated to have multiple biological functions, such as anti-HBV[9,24], inhibiting HIV infection[25], inhibiting SARS coronavirus[26]. Romero[24] confirmed that baicalin has a moderate ability to reduce HBV production and has no toxic effect on host cells, and this effect against HBV was confirmed by our findings[10] also. Moreover, it was reported that baicalin[25] at the noncytotoxic concentrations inhibited both T cell tropic (X4) and monocyte tropic (R5) HIV-1 Env protein mediated fusion with cells expressing CD4/CXCR4 or CD4/CCR5, and the presence of baicalin at the initial stage of HIV-1 viral adsorption blocked the replication of HIV-1 , resulting in an early definitive DNA replication cessation. Chen[27] found that baicalin had antiviral activity against 10 clinical isolates of SARS coronavirus by neutralization tests, which was confirmed by plaque reduction assays. Jang[28] reported that baicalin had a protective effect against acetaminophen-induced hepatotoxicity in mice and showed that the effects might be due to a block of the bioactivity of acetaminophen by inhibiting the cytochrome P450 2E1 expression. As an antioxidant flavonoid in vitro baicalin has a strong antioxidant activity toward reactive oxygen species (ROS), including hydroxyl radical (OH·), superoxide anions (O2·) and peroxynitrite (ONOO2), and inhibits lipid peroxidation, promoting the repair of DNA single strand breakage caused by H2O2 in cultured NIH3T3 fibroblasts[29]. It has long been accepted that Sho-saiko-to functions as a potent anti-hepatic fibrosis agent, and Japanese investigators confirmed that the active components of Sho-saiko-to are baicalin and baicalein of flavonoids, and the chemical structures of baicalin and baicalein are very similar to silybinin, which shows anti-fibrogenic activities[30,31].
The mechanism of hepatic fibrosis is too complicated for a single drug to resolve. A drug or drug compounds with multi-effect-pathways and multi-effect-targets may have better efficacy than a single drug alone, and could change the current predicament in the therapy of hepatic fibrosis[19]. Continued progress is essential in order to identify the determinants and dynamics of fibrosis reversibility, to discover additional targets for anti-fibrotic therapy, and to develop customized multi-drug regimens[32]. Taken together, the results from 2.2.15 cells show that the inhibitory effect of OB combination on HBV antigen secretion and HBV DNA replication is better than that of oxymatrine alone; furthermore, the results from HSC-T6 also show that the suppressing effect of OB combination on α SMA and type I collagen expression is more effective than that of oxymatrine. This study indicates that the addition of baicalin to oxymatrine can strengthen the treatment effect of oxymatrine in vitro. However, this finding needs to be verified in in vivo studies.
S- Editor Pan BR L- Editor Zhu LH E- Editor Ma N
| 1. | Rokuhara A, Sun X, Tanaka E, Kimura T, Matsumoto A, Yao D, Yin L, Wang N, Maki N, Kiyosawa K. Hepatitis B virus core and core-related antigen quantitation in Chinese patients with chronic genotype B and C hepatitis B virus infection. J Gastroenterol Hepatol. 2005;20:1726-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Cheng Y, Zhang M, Liu C, Wu X, Zhang Q. Effect of Oxymatrine on collagen synthesis and expression of TGF-β1 in an immune hepatic fibrosis model. Zhongxiyi Jiehe Ganbing Zazhi. 2001;11:210-212. |
| 3. | Cheng Y, Zhang M, Li H, Wu X, Zhang Q. Study on the mechanism of oxymatrine on immune hepatic fibrosis by Concanavalin A in BALB/c mice. Xiandai Shiyong Yixue. 2001;13:14-16. |
| 4. | Friedman SL. Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol. 2004;1:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 386] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 5. | Zavaglia C, Airoldi A, Pinzello G. Antiviral therapy of HBV- and HCV-induced liver cirrhosis. J Clin Gastroenterol. 2000;30:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Kim JW, Lee HS, Woo GH, Yoon JH, Jang JJ, Chi JG, Kim CY. Fatal submassive hepatic necrosis associated with tyrosine-methionine-aspartate-aspartate-motif mutation of hepatitis B virus after long-term lamivudine therapy. Clin Infect Dis. 2001;33:403-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Liaw YF, Chien RN, Yeh CT, Tsai SL, Chu CM. Acute exacerbation and hepatitis B virus clearance after emergence of YMDD motif mutation during lamivudine therapy. Hepatology. 1999;30:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 403] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 8. | Lu LG, Zeng MD, Mao YM, Li JQ, Wan MB, Li CZ, Chen CW, Fu QC, Wang JY, She WM. Oxymatrine therapy for chronic hepatitis B: a randomized double-blind and placebo-controlled multi-center trial. World J Gastroenterol. 2003;9:2480-2483. [PubMed] |
| 9. | Mao YM, Zeng MD, Lu LG, Wan MB, Li CZ, Chen CW, Fu QC, Wang JY, She WM, Cai X. Capsule oxymatrine in treatment of hepatic fibrosis due to chronic viral hepatitis: a randomized, double blind, placebo-controlled, multicenter clinical study. World J Gastroenterol. 2004;10:3269-3273. [PubMed] |
| 10. | Cheng Y, Ping J, Xu HD, Fu HJ, Zhou ZH. The inhibitory effect of oxymatrine-baicailin compound on hepatitis B viral antigens secretion in HepG2.2.2.15 cells. Zhongguo Yaolixue Tongbao. 2006;22:257-261. |
| 11. | Sells MA, Chen ML, Acs G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci USA. 1987;84:1005-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 939] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 12. | Vogel S, Piantedosi R, Frank J, Lalazar A, Rockey DC, Friedman SL, Blaner WS. An immortalized rat liver stellate cell line (HSC-T6): a new cell model for the study of retinoid metabolism in vitro. J Lipid Res. 2000;41:882-893. [PubMed] |
| 13. | Zhou ZH, Cheng Y, Xu HD, Fu HJ, Ping J, inventors . Shanghai Kairuisi Biotechnological Co. Ltd., assignee. A drug-compound comprising oxymatrine and baicalin. China National Invention Patent Application No. 200510029111.6, 2005 Aug 26. . |
| 14. | Cheng Y, Wu X, Weng X, Liu C, Li H, Zhang Q. The fluctuation of cytokine mRNA expression level of a novel T-cell-mediated immune hepatic fibrosis model in Balb/c mice. Zhonghua Chuanranbing Zazhi. 2002;20:21-25. |
| 15. | Cheng Y, Dharancy S, Malapel M, Desreumaux P. Hepatitis C virus infection down-regulates the expression of peroxisome proliferator-activated receptor alpha and carnitine palmitoyl acyl-CoA transferase 1A. World J Gastroenterol. 2005;11:7591-7596. [PubMed] |
| 16. | Gish RG. Current treatment and future directions in the management of chronic hepatitis B viral infection. Clin Liver Dis. 2005;9:541-565, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Dong Y, Xi H, Yu Y, Wang Q, Jiang K, Li L. Effects of oxymatrine on the serum levels of T helper cell 1 and 2 cytokines and the expression of the S gene in hepatitis B virus S gene transgenic mice: a study on the anti-hepatitis B virus mechanism of oxymatrine. J Gastroenterol Hepatol. 2002;17:1299-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1597] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 19. | Cheng Y, Ping J, Liu C, Tan YZ, Chen GF. Study on effects of extracts from Salvia Miltiorrhiza and Curcuma Longa in inhibiting phosphorylated extracellular signal regulated kinase expression in rat's hepatic stellate cells. Chin J Integr Med. 2006;12:207-211. [PubMed] |
| 20. | Shi GF, Li Q. Effects of oxymatrine on experimental hepatic fibrosis and its mechanism in vivo. World J Gastroenterol. 2005;11:268-271. [PubMed] |
| 21. | Wu CS, Piao XX, Piao DM, Jin YR, Li CH. Treatment of pig serum-induced rat liver fibrosis with Boschniakia rossica, oxymatrine and interferon-alpha. World J Gastroenterol. 2005;11:122-126. [PubMed] |
| 22. | Xiang X, Wang G, Cai X, Li Y. Effect of oxymatrine on murine fulminant hepatitis and hepatocyte apoptosis. Chin Med J (Engl). 2002;115:593-596. [PubMed] |
| 23. | Zhu XH, Qiu YD, Shen H, Shi MK, Ding YT. Effect of matrine on Kupffer cell activation in cold ischemia reperfusion injury of rat liver. World J Gastroenterol. 2002;8:1112-1116. [PubMed] |
| 24. | Romero MR, Efferth T, Serrano MA, Castaño B, Macias RI, Briz O, Marin JJ. Effect of artemisinin/artesunate as inhibitors of hepatitis B virus production in an "in vitro" replicative system. Antiviral Res. 2005;68:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 25. | Li BQ, Fu T, Dongyan Y, Mikovits JA, Ruscetti FW, Wang JM. Flavonoid baicalin inhibits HIV-1 infection at the level of viral entry. Biochem Biophys Res Commun. 2000;276:534-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 159] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Wu JA, Attele AS, Zhang L, Yuan CS. Anti-HIV activity of medicinal herbs: usage and potential development. Am J Chin Med. 2001;29:69-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Chen F, Chan KH, Jiang Y, Kao RY, Lu HT, Fan KW, Cheng VC, Tsui WH, Hung IF, Lee TS. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31:69-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 385] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 28. | Jang SI, Kim HJ, Hwang KM, Jekal SJ, Pae HO, Choi BM, Yun YG, Kwon TO, Chung HT, Kim YC. Hepatoprotective effect of baicalin, a major flavone from Scutellaria radix, on acetaminophen-induced liver injury in mice. Immunopharmacol Immunotoxicol. 2003;25:585-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Chen X, Nishida H, Konishi T. Baicalin promoted the repair of DNA single strand breakage caused by H2O2 in cultured NIH3T3 fibroblasts. Biol Pharm Bull. 2003;26:282-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Shimizu I. Antifibrogenic therapies in chronic HCV infection. Curr Drug Targets Infect Disord. 2001;1:227-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Shimizu I. Sho-saiko-to: Japanese herbal medicine for protection against hepatic fibrosis and carcinoma. J Gastroenterol Hepatol. 2000;15 Suppl:D84-D90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 89] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Friedman SL, Bansal MB. Reversal of hepatic fibrosis -- fact or fantasy. Hepatology. 2006;43:S82-S88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 283] [Article Influence: 14.9] [Reference Citation Analysis (0)] |