Published online Aug 21, 2006. doi: 10.3748/wjg.v12.i31.5005
Revised: May 20, 2006
Accepted: May 25, 2006
Published online: August 21, 2006
AIM: To investigate the role of intercellular adhesion molecule-1 (ICAM-1) and its counter receptors LFA-1 and Mac-1 in acute pancreatitis (AP).
METHODS: SD rats were allocated to AP group and control group randomly (25 rats each). AP was induced by infusion of 5% chenodeoxycholic acid into the pancreatic duct, followed by ligation of pancreatic duct. The rats were sacrificed at 1, 3, 6, 12 and 24 h after induction of pancreatitis. Five rats were sacrificed at one time point in the two groups before the blood and specimens from pancreas and lung were obtained. Serum amylase and ascitic fluid were measured at each time point. Expression of ICAM-1 at different time points was assessed by immunohistochemistry in pancreas and lung, and the expression of LAF-1 and Mac-1 on neutrophils at different time points was detected by flow cytometer.
RESULTS: Induction of AP was confirmed by the serum levels of amylase and histological studies. The expression of ICAM-1 in pancreas increased significantly than that in the control group at all time points (P < 0.05 or P < 0.01), as well as the expression in lung except at 1 h. The expression of LFA-1 and Mac-1 on neutrophil in blood increased significantly in AP group than that in control group at several time points (P < 0.05 or P < 0.01). The amount of ascitic fluid and serum amylase level of AP group increased significantly than that of control group at all time points (P < 0.05 or P < 0.01). Parallel to these results, a significant neutrophil infiltration was found in pancreas and lung tissues of AP group rats.
CONCLUSION: Our findings suggest the important role for ICAM-1, LFA-1 and Mac-1 in mediating the development of AP from a local disease to a systemic illness. Upregulation of ICAM-1, LFA-1, Mac-1 and subsequent leukocyte infiltration appear to be significant events of pancreatic and pulmonary injuries in AP.
- Citation: Sun W, Watanabe Y, Wang ZQ. Expression and significance of ICAM-1 and its counter receptors LFA-1 and Mac-1 in experimental acute pancreatitis of rats. World J Gastroenterol 2006; 12(31): 5005-5009
- URL: https://www.wjgnet.com/1007-9327/full/v12/i31/5005.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i31.5005
Acute pancreatitis (AP) is a disease of variable severity. Approximately 80% of patients have a relatively mild attack that resolves with few or no complications. The mortality rate is low. However, 20% of patients in whom pancreatic necrosis develops may incur systemic complications with a mortality rate as high as 40%[1]. Recent investigations have established that one of the earliest pathophysiologic events in pancreatitis is the colocalization of acinar cell organelles containing digestive and lysosomal enzymes, resulting in premature intracellular activation of proteases[2,3]. The individual pancreatic cell injury becomes magnified and propagated by inducing impaired microcirculation, leukocyte adhesion, and leukocyte infiltration. These become central events in the pathogenesis of pancreatic necrosis and extrapancreatic complications[4,5]. Organ dysfunction occurs in one of four patients with acute pancreatitis, and 60% of them die from pulmonary damage[6].
Intercellular adhesion molecule-1 (ICAM-1) is expressed on endothelial cells and is responsive to numerous inflammatory mediators[7]. It mediates both leukocyte adhesion and migration through the endothelium into tissues[8]. Lymphocyte function-associated antigen 1 (LFA-1) and Mac-1 are the counter receptors of ICAM-1, both of which are expressed on leukocytes and are related with the above mediating activities of ICAM-1[9].
The aim of the present study was to analyze the mechanism of upregulation and levels of expression of ICAM-1 in the pancreas and lung tissues of AP, to detect the expression of LFA-1 and Mac-1 on neutrophils, and to explore the role of these adhesion molecules in the development of AP from a local disease to a systemic illness.
Sprague-Dawley rats (250 to 300 g) were fasted 12 h before the experiment but were allowed free access to water. Fluothane, oxygen and nitrous oxide were used in surgical anesthesia (2 L/min). All rats underwent a 2-cm-long midline laparotomy, and the pancreatic duct was visualized entering the duodenum. The duct was cannulated and AP was induced by retrograde intraductal injection of 5% chenodeoxycholic bile acid (0.1 mL/kg, Wako Pure Chemical Industries, Ltd., Osaka, Japan) for 2 min. After injection, the pancreatic duct was ligated and the abdomen was closed.
A total of 50 rats were allocated to control group and AP group (25 rats in each). The rats of control group only received laparotomy without retrograde intraductal injection of 5% chenodeoxycholic bile acid of pancreas and the pancreatic duct ligation. Blood samples (2 mL) were drawn through inferior vena cava. Pancreatic and lung tissues were removed and processed as indicated below before the rats were killed at 1, 3, 6, 12 and 24 h after induction of pancreatitis (n = 5 per group). Ascitic fluid was quantified by estimation of weight of towels after absorption of the ascitic fluid from the abdominal cavity (assuming 1 g = 1 mL).
One milliliter blood was sampled to measure amylase in plasma using the RaBA-SUPER (Kyoto First Science Ltd., Kyoto, Japan) kit.
For immunohistochemical staining, pancreatic and lung tissues were immediately snap-frozen in Freon precooled in liquid nitrogen and embedded in OCT embedding medium (Tissue-Tek, Miles Inc, Elkhart, USA). Cryostat sections (5 μm) were fixed in acetone for 10 min, and endogenous peroxidase activity was quenched by incubation of methanol with 0.3% H2O2 for 5 min. After rinsing with 0.02 mol PBS, the sections were incubated with primary anti-ICAM-1 antibody (Santa Cruz Biotechnology, Inc., California, USA) diluted at 1:400 in antibody dilution (Dako Corporation, Carpinteria, CA, USA) for 10 min in moist chamber at room temperature. Unbound antibodies were washed from the tissue three times in PBS for 3 min, incubated for 10 min with the secondary biotinylated anti-mouse IgG antibody (LSAB II kit, Dako Corporation, Carpinteria, CA, USA), and then washed three times for 3 min. The sections were incubated with the LSAB reagent (LSAB® 2 System, HRP KIT, DAKO Corporation, Carpinteria, CA, USA) for 10 min, washed and developed in DAB solution. Finally, the slides were fixed in alcohol, counter-stained 3 times with hematoxylin for 10 min, and covered with DAKO Glycergel. NIH Image 1.61/ppc software was used to detect the density of positive area of slides.
Blood (1 mL) was anticoagulated with 2% EDTA (0.2 mL). Leukocyte-rich plasma was obtained by centrifugation for 10 min at 1500 r/min. The erythrocytes were then lysed 15 min with 4 mL of hemolysin twice. The leukocyte pellet was washed once with PBS and resuspended with 1 mL of PBS. Leukocytes suspensions (100 μL) were mixed with 20 μL of FITC-labeled monoclonal antibody anti-LFA-1 (CD11a/CD18) (BD Pharmingen, San Diego, CA, USA), FITC-labeled anti-Mac-1 (CD11b/CD18) (BD Pharmingen, San Diego, CA, USA), and FITC-labeled IgG (Sigama, Saint Louis, Missouri, USA) that acted as a nonspecific control antibody. It was then incubated at 4°C for 30 min, washed twice in ice-cold PBS, resuspended in 1 mL of PBS and kept on ice until analyzed. Flow cytometry was performed on an FACScan (Coulter Epics XL, USA). Data were collected for forward scatter, side scatter, and fluorescence on the FL1 channel (530 nm) with Coulter Elite software. Cells were analyzed with a gate setting for neutrophils and twenty thousand cell counts were accumulated for analysis. Green fluorescence (FL1) was determined and mean fluorescence intensity (MFI) was calculated. The nonspecific binding (IgG) in control group and AP group was measured at MFI values of 2.81 (0.57) and 3.74 (0.97) units, respectively.
Results are expressed as means and standard errors of the mean. Differences between AP and control groups were judged by using the Student’s t test for parametric data. P values of less than 0.05 were considered significant.
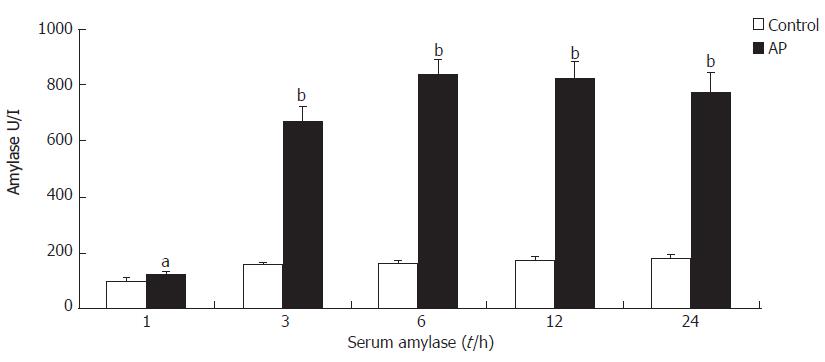
In AP group rats, there was a marked increase in serum amylase from 1 to 24 h with a peak value after 6 h (Figure 1).
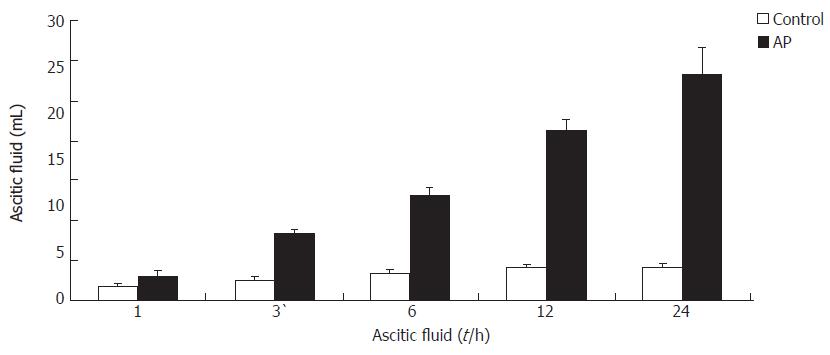
The amount of ascitic fluid in AP group increased significantly than that in control group at the same time point (Figure 2).
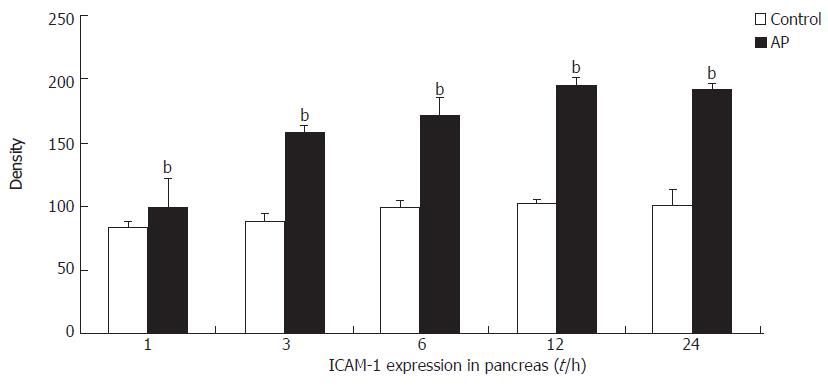
A low level of ICAM-1 expression was observed in the pancreas and lung tissues in control rats. ICAM-1 expression in the pancreas of AP rats was significantly increased above control levels at 1 h, and continued to increase until 12 h and remained elevated at that level for the whole observation period (24 h) (Figure 3).
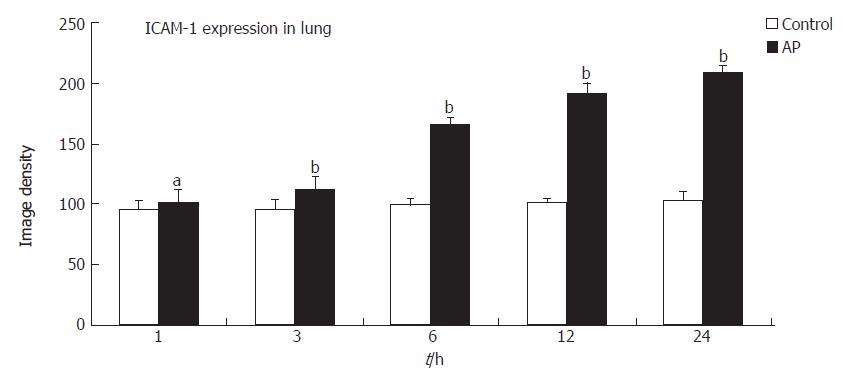
ICAM-1 expression in the lung of AP rats was significantly increased above control levels at 3 h, and continued to increase until 24 h (Figure 4). Immunohistochemical analysis showed that overexpression of ICAM-1 was localized in small venules and capillaries.
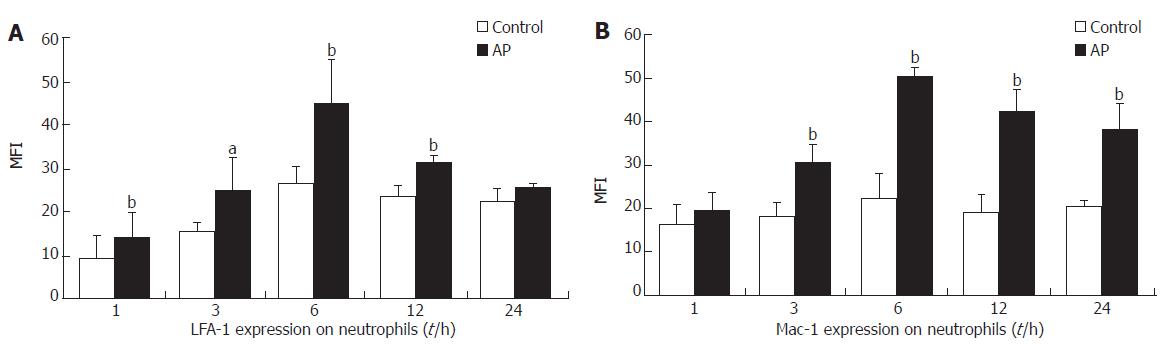
The expression of LFA-1 and Mac-1 on leukocytes in AP group increased significantly than that in the control group (Figure 5).
Intra-acinar cell activation of digestive enzyme zymogens is generally believed to be an early event in pancreatitis. Most researchers, however, believe that subsequent nonacinar cell phenomena, including the activation and recruitment of inflammatory cells, play a critical role in determining the ultimate severity of the disease. Nonacinar cell phenomena including the local generation and release of proinflammatory factors and activation of circulating inflammatory cells are also believed to couple pancreatic injury to distant organ complications, such as lung injury.
Leukocyte sequestration within areas of injury and inflammation is a multistep process that begins with leukocyte activation. It involves the adhesion of circulating activated inflammatory cells to microvascular endothelial surfaces, and culminates in the transmigration of those cells across the endothelial barrier and into the involved tissue. Currently available evidence indicates that the adhesion of activated leukocytes, including neutrophils, to endothelial surfaces results from the interaction of leukocyte surface proteins, such as CD11/CD18, with endothelial cell surface adhesion molecules such as ICAM-1.
Surprisingly, little attention has been paid to probing the relationship between the ICAM-1 and its counter receptors (LFA-1, Mac-1). Furthermore, the time course of LFA-1 and Mac-1 expression in severe acute pancreatitis has not been studied. We serially investigated LFA-1 and Mac-1 expression on circulatory neutrophils and ICAM-1 expression in pancreas and lung tissues of AP rats in the current study.
ICAM-1 is an adhesion molecule expressed on endothelial cells that mediates both firm adhesion of leukocytes to the endothelium and transmigration and infiltration of marginated leukocytes into the organs[7]. Endothelial ICAM-1 is enhanced by hypoxia[10] and cytokines, including, among others, interleukin-6, interleukin-8 and tumor necrosis factor-α[2,7]. These inflammatory mediators, oxygen free radicals, and cytokines respond early in the pathogenesis process of acute pancreatitis[2,11,12].
ICAM-1 is a counter receptor for LFA-1(CD11a/CD18) and Mac-1(CD11b/CD18). The interaction between ICAM-1 and CD11/CD18 is a major determinant of leukocyte adhesion to endothelial cells[13], a phenomenon that is itself a prelude to leukocyte transmigration across the endothelial barrier and into areas of inflammation.
The multistep model of leukocyte emigration provides a conceptual framework describing the molecular recognition mechanism that leads from cell capture to adhesion strengthening and transmigration. Neutrophils use selectins to tether to inflamed endothelium and subsequently arrest at septic sites in the vasculature through binding of the β2-integrins[14,15]. The β2-integrins family members LFA-1 and Mac-1 are involved in firm adhesion of neutrophils to endothelial cells[16]. Intravital microsopy in mice and rats has shown that CD11a and CD11b each contribute significantly to firm adhesion, and concurrent blocking of both β2-integrins family is required for complete inhibition of human and canine neutrophil transmigration[17-19]. Furthermore, the adhesion complex CD11b/CD18, induced by inflammatory cytokines, has been demonstrated to play a crucial role in neutrophil-induced tissue damage by enhancing neutrophil migration and adhesiveness[20].
We probed the expression of adhesion molecules such as ICAM-1 and its counter receptors, LFA-1 and Mac-1 in AP. We found expression of ICAM-1 was markedly upregulated in AP, as well as the expression of LFA-1 and Mac-1 on neutrophils. ICAM-1 is also upregulated in organs beyond the pancreas in severe pancreatitis. Our experiments showed an increased expression of ICAM-1 in the endothelium of venules and capillaries within the lungs at 6 h in AP, followed by massive leukocyte infiltration. The results of this study imply the important roles for ICAM-1 and its counter receptors LFA-1 and Mac-1 in the pathogenesis of pancreatic and systemic injury in acute necrotizing pancreatitis. The current studies are consistent with the concept that expression of ICAM-1, LFA-1 and Mac-1 in the pancreas during AP is a critical link in the development of tissue injury and organ dysfunction. Although overexpression of inflammatory mediators do play an important role in pathogenesis but the molecular mechanisms of the whole cascade, i.e. cell surface molecules, signaling into the cell and resulting response of the cell has still not been worked out. It may be possible to prevent tissue injury and necrosis and thereby to minimize the possibility for subsequent secondary infection by administering antibodies or other drugs in AP. Further studies are in progress to define the window of opportunity.
S- Editor Wang J L- Editor Zhu LH E- Editor Bi L
| 1. | Steer ML. Etiology and pathophysiology of acute pancreatitis. The pancreas: biology, pathophysiology, and disease. New York: Raven 1993; 581-592. |
| 2. | Kingsnorth A. Role of cytokines and their inhibitors in acute pancreatitis. Gut. 1997;40:1-4. [PubMed] |
| 3. | Bhatia M. Inflammatory response on the pancreatic acinar cell injury. Scand J Surg. 2005;94:97-102. [PubMed] |
| 4. | Klar E, Messmer K, Warshaw AL, Herfarth C. Pancreatic ischaemia in experimental acute pancreatitis: mechanism, significance and therapy. Br J Surg. 1990;77:1205-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 172] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Werner J, Dragotakes SC, Fernandez-del Castillo C, Rivera JA, Ou J, Rattner DW, Fischman AJ, Warshaw AL. Technetium-99m-labeled white blood cells: a new method to define the local and systemic role of leukocytes in acute experimental pancreatitis. Ann Surg. 1998;227:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Mann DV, Hershman MJ, Hittinger R, Glazer G. Multicentre audit of death from acute pancreatitis. Br J Surg. 1994;81:890-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 128] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4705] [Cited by in RCA: 4727] [Article Influence: 135.1] [Reference Citation Analysis (0)] |
| 8. | Pober JS, Cotran RS. The role of endothelial cells in inflammation. Transplantation. 1990;50:537-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 576] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 9. | Smith CW. Endothelial adhesion molecules and their role in inflammation. Can J Physiol Pharmacol. 1993;71:76-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 117] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Zünd G, Uezono S, Stahl GL, Dzus AL, McGowan FX, Hickey PR, Colgan SP. Hypoxia enhances induction of endothelial ICAM-1: role for metabolic acidosis and proteasomes. Am J Physiol. 1997;273:C1571-C1580. [PubMed] |
| 11. | Schoenberg MH, Büchler M, Gaspar M, Stinner A, Younes M, Melzner I, Bültmann B, Beger HG. Oxygen free radicals in acute pancreatitis of the rat. Gut. 1990;31:1138-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 146] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Norman JG, Franz MG, Fink GS, Messina J, Fabri PJ, Gower WR, Carey LC. Decreased mortality of severe acute pancreatitis after proximal cytokine blockade. Ann Surg. 1995;221:625-631; discussion 631-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Kelly KJ, Williams WW Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, Bonventre JV. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97:1056-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 577] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 14. | Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1551] [Cited by in RCA: 1512] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 15. | Konstantopoulos K, McIntire LV. Effects of fluid dynamic forces on vascular cell adhesion. J Clin Invest. 1997;100:S19-S23. [PubMed] |
| 16. | Smith CW, Marlin SD, Rothlein R, Toman C, Anderson DC. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989;83:2008-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 818] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 17. | Kurose I, Anderson DC, Miyasaka M, Tamatani T, Paulson JC, Todd RF, Rusche JR, Granger DN. Molecular determinants of reperfusion-induced leukocyte adhesion and vascular protein leakage. Circ Res. 1994;74:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 213] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Furie MB, Tancinco MC, Smith CW. Monoclonal antibodies to leukocyte integrins CD11a/CD18 and CD11b/CD18 or intercellular adhesion molecule-1 inhibit chemoattractant-stimulated neutrophil transendothelial migration in vitro. Blood. 1991;78:2089-2097. [PubMed] |
| 19. | Entman ML, Youker K, Shoji T, Kukielka G, Shappell SB, Taylor AA, Smith CW. Neutrophil induced oxidative injury of cardiac myocytes. A compartmented system requiring CD11b/CD18-ICAM-1 adherence. J Clin Invest. 1992;90:1335-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 221] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Jaeschke H, Farhood A, Smith CW. Neutrophil-induced liver cell injury in endotoxin shock is a CD11b/CD18-dependent mechanism. Am J Physiol. 1991;261:G1051-G1056. [PubMed] |