Published online Aug 21, 2006. doi: 10.3748/wjg.v12.i31.4996
Revised: March 6, 2006
Accepted: March 13, 2006
Published online: August 21, 2006
AIM: To pharmacologically modulate Th polarization in the ileum exposed to ionizing radiation by using the immuno-modulatory/apoptotic properties of Caffeic Acid Phenethyl Ester (CAPE).
METHODS: Rats received CAPE (30 mg/kg) treatment ip 15 min prior to intestinal 10 Gy γ-irradiation and once a day for a 6 d period after irradiation. Expression of genes implicated in Th differentiation in ileal mucosa (IL-23/IL-12Rβ2), Th cytokine responses (IFN-γ, IL-2, IL-4, IL-13), Th migratory behaviour (CXCR3, CCR5, CCR4), Th signalling suppressors (SOCS1, SOCS3), transcription factor (T-Bet, GATA-3) and apoptosis (FasL/Fas, TNF/TNFR, XIAP, Bax, caspase-3) was analyzed by RT-PCR 6 h and 7 d post-irradiation. CD4+ and TUNEL positive cells were visualized by immunostaining.
RESULTS: The expression of Th1-related cytokine/chemokine receptors (IFN-γ, IL-2, CXCR3, CCR5) was repressed at 7 d post-irradiation while Th2 cell cytokine/chemokines (IL-4, IL-13, CCR4) were not repressed or even upregulated. The irradiation-induced Th2 profile was confirmed by the upregulation of both Th2-specific transcription factor GATA-3 and SOCS3. Although an apoptosis event occurred 6 h after 10 Gy of intestinal γ-irradiation, apoptotic mediator analysis showed a tendency to apoptotic resistance 7 d post-irradiation. CAPE amplified apoptotic events at 6h and normalized Bax/ FasL expressions at 7 d.
CONCLUSION: CAPE prevented the ileal Th2 immune response by modulating the irradiation-influenced cytokine environment and apoptosis.
- Citation: Grémy O, Benderitter M, Linard C. Caffeic acid phenethyl ester modifies the Th1/Th2 balance in ileal mucosa after γ-irradiation in the rat by modulating the cytokine pattern. World J Gastroenterol 2006; 12(31): 4996-5004
- URL: https://www.wjgnet.com/1007-9327/full/v12/i31/4996.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i31.4996
Radiation-induced acute enteritis is the major dose-limiting complication in the radiotherapy for cancers in the abdominal region[1]. Irradiation injuries involve cytokine pathways[2] that trigger an inflammatory process causing clinical symptoms (diarrhea, dismotility). The immune-mediated pathologic development is the consequence of sustained local cytokine production. In particular, the modification of the CD4+ helper T (Th) cell polarization induced by abdominal irradiation has not been investigated and these cells may play a primary role in irradiation injury. Crohn’s disease and TNBS-induced colitis are both Th1-like forms of gut inflammation[3] whereas infection with Schistosoma is associated with a Th2 immune response[4].
Activated CD4+ Th cells have been divided into at least two major exclusive T cell subsets according to their cytokine profile. The cytokine environment has been proposed as the major variable influencing Th development[5]. In the presence of IL-12, activated Th cells differentiate into Th1 cells, secreting predominantly IFN-γ and IL-2 which promote delayed-type hypersensitivity responses and pro-inflammatory responses. In contrast, the Th cells that are activated in the presence of IL-4 differentiate into Th2 cells, producing mainly IL-4 and IL-13 and promote humoral and allergic responses[6]. In addition, IL-4 inhibits the expression of the β2 subunit of the IL-12 receptor, thereby preventing a Th1-type response, whereas IFN-γ stimulates it[7]. Thus, IL-12Rβ2 expression is preferentially found in Th1 cells and maintains responsiveness to IL-12, whereas Th2 cell development is accompanied by a loss of this receptor subunit. Th1 and Th2 cells were originally distinguished from each other by their partially specific profile of cytokine expression. Analysis of such in vivo polarized Th cells has revealed differential sets of gene expression, including specific chemokine/chemokine receptors. Typically, Th1 cells migrate to inflammatory sites in response to CXCR3 and CCR5 ligands[8]. Likewise, CCR4 is preferentially expressed on Th2 cells. The restricted expression of cytokines by polarized Th cells arises from a differential expression of transcription factors. T-Bet, expressed specifically in Th1, mediates IFN-γ production and GATA-binding protein 3 (GATA-3) plays a pivotal role in Th2 phenotype development and in Th1 phenotype inhibition[9]. However, recent studies have shown that cytokine signalling is under a negative feedback regulation by proteins called suppressors of cytokine signalling (SOCS). Thus, the differentiation toward the Th1 or Th2 pathway may be mediated in part by the selective repression of IL-12 or IL-4 signalling pathways[10].
Inhibition of pro-inflammatory molecules provides a logical therapeutic option for patients with abnormal immune cell activation[11]. This concept should be re-evaluated in light of evidence that immune activation is also regulated by apoptosis. Because programmed cell death controls the life span of cells, an apoptosis defect could result in an inappropriately long cell survival and a state of uncontrolled inflammation leading to chronic and autoimmune diseases[12]. This may be the case in Crohn disease, where abnormal mucosal T cell reactivity is well documented[11]. Medical or accidental exposure to radiation could lead to an impairment of specific immune responsiveness by apoptotic death of resting lymphocytes. The transcription factor NF-κB is a key regulator of genes implicated in the immune/inflammatory response[13]. Recently, we have shown that abdominal irradiation induced an inflammatory process involving NF-κB activation[3]. NF-κB also regulates the expression of many genes involved in the control of cell proliferation and cell death via extrinsic factors [TNF-α, Fas Ligand (FasL), IL-2], specific receptors [TNF and Fas receptors (TNFR, Fas)] and intrinsic mediators (XIAP, Bax)[14]. Targeting of inducible extrinsic factors has been proposed as a method for constraining immune responses by selectively killing activated T cells or inhibiting differentiation[15].
The modulation of NF-κB action by specific drugs is primarily based on cytokine synthesis. However, little is known about the effects of these drugs on Th1 or Th2-derived cytokine production patterns, on their specific transcription factors (T-Bet, GATA-3) and on chemokine receptors CXCR3/CCR5 (on Th1 cells) or CCR4 (on Th2 cells). Here, using abdominal γ-irradiation where both type-1 and type-2 cells can be activated, the pattern of gene expression was analysed in the ileum mucosa. To test the hypothesis that defective apoptosis is involved in inflammation process[16] and orients the immune response following irradiation, we used the apoptotic and immune regulation properties of Caffeic Acid Phenethyl Ester (CAPE), an active phenolic compound inhibitor of NF-κB[17] to modulate the irradiation-influenced cytokine environment and the Th polarization at the dose previously used[18]. This study was performed at 6 h post-irradiation where apoptotic events occurred, and at one week (d 7) corresponding to the restoration of the intestinal morphological structure.
Male Wistar rats (Elevage Janvier, France), weighing 200-250 g, were housed with ad libitum food and water. All experiments were conducted in accordance with the French regulations for animal experimentation (Ministry of Agriculture Order No. 87-848, October 19, 1987). Anesthetized rats received a single abdominal dose of 10 Gy γ-irradiation (60Co source). Control rats were sham-irradiated. Rats were divided into four groups (n = 6): controls, controls treated with CAPE, irradiated untreated, and irradiated CAPE treated. Rats were injected intraperitoneally with CAPE (30 mg/kg in saline containing 200 g/L Tween 80) 10 min before irradiation and once a day for 6 d[18]. The animals were sacrificed and the ileal tissues were dissected at 6 h and 7 d after exposure. Entire ileum specimens were taken for immunostaining and only mucosa layers, separated from the muscularis by scraping, were kept at -80°C for protein extraction and RT-PCR.
Ileal tissue samples were washed in PBS and then fixed in 40 g/L buffered formaldehyde solution. After dehydration, specimens were embedded in paraffin and sectioned (5 μm).
CD4+ detection: Immunohistochemistry was performed to assess the presence of CD4 phenotype cells. Briefly, deparaffinized and rehydrated tissue sections were treated with 3 mL/L hydrogen peroxide in methanol to inhibit the endogenous peroxidase activity. Sections were incubated in protein blocking serum (Dako, USA) before treatment for 90 min at room temperature with the monoclonal anti-CD4 antibody (ab6413; 1/100 dilution, Abcam). Slides were then rinsed in 50 mmol/L Tris/HCl, 3 g/L NaCl and 1g/L Tween-20. The secondary reagent was the Vector Elite ABC kit (Dako).
Apoptotic cell detection: Apoptotic cells were visualized by the terminal deoxynucleotidyltransferase (TdT)-mediated dUTP-biotin nick-end labelling assay (TUNEL) using the In Situ Cell Death Detection kit (Roche Molecular Biochemicals, France) according to the manufacturer’s instructions. Briefly, deparaffinized and rehydrated ileum tissue sections were incubated in proteinase K (20 mg/L in 10 mmol/L Tris-HCl, pH 7.6) for 10 min at 37°C. Endogenous peroxidase activity was quenched with blocking solution (3 g/L H2O2 in methanol). Sections were exposed to the TUNEL reaction mixture at 37°C for 1 h. After washing in PBS buffer, POD (peroxidase) was added and allowed to react for 30 min at 37°C. For both staining methods, the slides were treated with a NovaRED™ kit (Vector Laboratories Inc, Burlingame, CA) for colour development and counterstained with Meyer’s hemalum.
Mucosal protein extracts were prepared by a Dounce homogenization protocol from 50 mg of tissue in a hypotonic buffer (25 mmol/L HEPES, pH 7.5, 5 mmol/L MgCl2, 1 mmol/L EGTA, 1 mmol/L PMSF, 5 mg/L aprotinin and leupeptin). After centrifugation of the homogenates, the supernatants were collected. Five hundred μg of extracted protein was incubated with 100 μmol/L of the synthetic substrate Ac-DEVD-pNA in 500 µl of 25 mmol/L HEPES (pH 7.5) containing 200 ml/L glycerol and protease inhibitors. After 20 min of reaction at 37°C, the caspase-3-like activity was assayed by measuring the absorbance at 405 nm.
Real-time quantitative RT-PCR was used to measure the expression of Th1 (IL-23, IL-12Rβ2, IFN-γ, IL-2, CXCR3, CCR5, T-Bet and SOCS1) or Th2-derived genes (IL-4, IL-13, CCR4, GATA-3, SOCS3), the cytokine TNF-α and its specific receptors (TNFR1, TNFR2), cytotoxic mediators (Fas/FasLigand, CTLA-4) and apoptotic genes (Bax, XIAP, caspase-3). Total RNA was prepared with the RNeasy total RNA isolation kit (Qiagen, France) according to the manufacturer’s instructions. RNA integrity was confirmed by electrophoresis on a denaturing agarose gel and ethidium bromide staining. One µg of total RNA was reverse transcribed with SuperScript II reverse transcriptase (Invitrogen, France) using random hexamers. IL-4 and IL-13 primers from the manufacturer were used to amplify first-strand cDNA with TaqMan technology (Applied Biosystems). PCR amplification of the other genes used SYBR PCR master Mix. The primer sequences (Table 1) have been designed by Primer Express software (Applied Biosystems). Optimized PCR used the ABI Prism 7000 Sequence detection system (Applied Biosystems). PCR fluorescent signals were normalized to the signal obtained from the housekeeping gene HPRT for each sample.
| Gene | Forward primer | Reverse primer |
| Th1 cytokines/receptors | ||
| IFN-γ | CACGCCGCGTCTTGGT | TCTAGGCTTTCAATGAGTGTGCC |
| IL-2 | ATTTTCCAGGCACTGAAGATGTTT | CCCCATGATGCTCACGTTTA |
| IL-23 | GCGTTCTCTTCTCCGTTCCA | TGCTCCGTGGGCAAAGAC |
| IL-12Rβ2 | GGCTGCATCCTCCATTACAGA | CTGCTTATTGGATGTGAGTTTTG |
| Transcription factors | ||
| T-Bet | TCCTGTCTCCAGCCGTTTCT | CGCTCACTGCTCGGAACTCT |
| GATA-3 | GGCGGCGAGATGGTACTG | TCTGCCCATTCATTTTATGGTAGA |
| Chemokine receptors | ||
| CXCR3 | AGGTCAGTGAACGTCAAGTGCTAG | CAAAAAGAGGAGGCTGTAGAGGA |
| CCR5 | CGGAACTTCTCCCCAACAAA | CTTTCTCTTCTGGACTCCCTACAACA |
| CCR4 | GCCTCCAAGACAGACTTCCTTG | AGCGTTCGGTTCTAGTTTCCAC |
| Apoptotic Extrinsic factor/receptors | ||
| TNF-α | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC |
| TNFR1 | TGCCACGCAGGATTCTTTCTAAG | TGGGGGTTTGTGACATTTGC |
| TNFR2 | CCCATCATTGAACCAAGCATCAC | AAGCAGGTCGCCAGTCCTAACATC |
| Fas | CGAATGCAAGGGACTGATAGC | TTCTGAGCAGTTGTTGTCGGTTT |
| FasL | AAGGCGGCCTTGTGATCA | TTGCAAGACTGACCCCGG |
| CTLA-4 | GTGGAACTCATGTACCCACCG | GCATGGTTCTGGATCGATGAC |
| Apoptotic Intrinsic factors | ||
| XIAP | GGTGCAAGAAGCTATACGAATGG | AGTTGCTCCCAGATGTTTGGAG |
| Bax | GCTGACCTTGGAGCAGCC | ATGTTGTTGTCCAGTTCATCGC |
| Caspase-3 | TGCGCCATGCTGAAACTG | CCTTCCGGTTAACACGAGTGA |
| Suppressors of Cytokine Signalling Proteins | ||
| SOCS1 | ACACTCACTTCCGCACCTTC | AGCAGCTCGAAAAGGCAGTC |
| SOCS3 | CCTCCAGCATCTTTGTC-GGAAGAC | TACTGGTCCAGGAACTCCCGAATG |
| Housekeeping | ||
| HPRT | GCTCGAGATGTCATGAAGGAGA | TCAGCGCTTTAATGTAATCCAGC |
Total proteins were obtained by homogenization of ileal samples in a cold RIPA buffer containing classical protease-inhibitor cocktail. Total proteins (40 μg) were boiled in SDS and mercaptoethanol buffer; then separated on a 120 g/L polyacrylamide gel (NuPAGE gels; Invitrogen, France) and electroblotted. Polyvinylidendifluoride membranes were incubated with a blocking solution (50 g/L skim milk in TPBS containing 1 mL/L Tween 20), washed with TPBS and incubated with rabbit polyclonal primary antibody directed against T-Bet for 1h at RT (dilution 1/200; Santa Cruz) or goat polyclonal GATA-3 overnight (dilution 1/100; Santa Cruz). After washing, immunodetection was performed with the respective horseradish-linked secondary antibody (1/2000; Amersham). Chemiluminescence was detected according to manufacturer’s protocol (ECL, Amersham Biosciences).
We used the comparative ΔΔCT-method[19] for relative mRNA quantification of target genes, normalized to an endogenous reference (HPRT) and a relevant non-irradiated control, equal to 2-ΔΔCT. ΔΔCT is the difference between the mean ΔCT (irradiated sample) and mean ΔCT (non-irradiated sample), where ΔCT is the difference between the mean CT(genes) and the mean CT(HPRT). Each sample was monitored for fluorescent dyes, and signals were regarded as significant if the fluorescence intensity significantly exceeded (10-fold) the standard deviation of the baseline fluorescence, defined as threshold cycles (CT).
All data are expressed as the mean ± SD error of the mean (SEM) for 6 animals. Comparisons between groups used Mann-Whitney test for non-paired data.
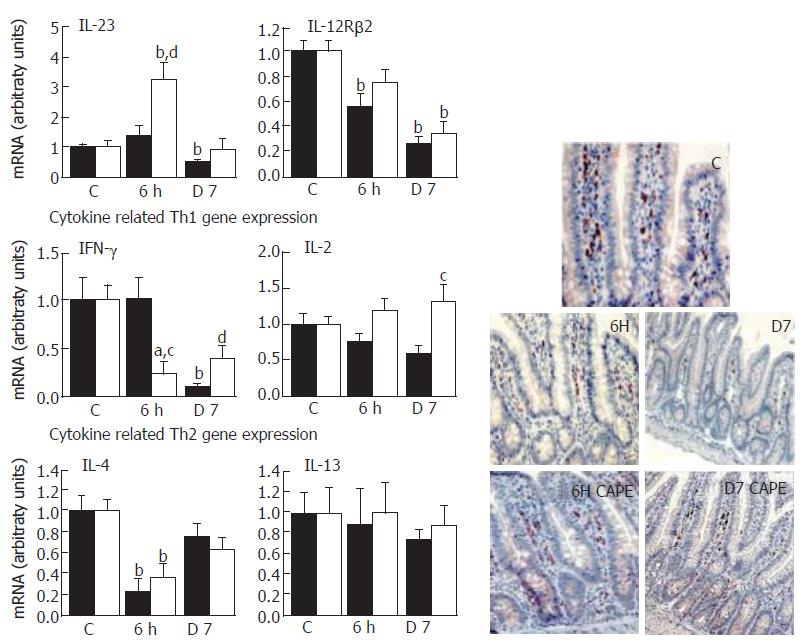
Activation and polarization of CD4+ Th cells was dependent on local dominant factors that control differentiation. IL-12 is a critical cytokine for polarizing naïve CD4+ T cells towards a Th1 dominance[7]. Moreover, the T-cell responsiveness to this mediator depends on expression of the inducible IL-12Rβ2 subunit of the IL-12 receptor[20]. IL-12Rβ2 is preferentially expressed by Th1 cells and is lost with Th2 cell development. From the analysis of IL-23 expression, the IL-12p40 form appears to be a key step in Th1 pathogenesis[21], since irradiation repressed it (P < 0.01) and it fell to below the levels of IL-12Rβ2 at d 7 (P < 0.01) (Figure 1). Significant evidence exists for a relationship between IFN-γ expression and IL-23/IL-12R signalling since Th1 commitment is enhanced by IFN-γ[22]. Accordingly, the irradiation-induced down-regulation of IL-23/IL-12Rβ2 (-50% and -80%) was correlated with a drastically low level of IFN-γ expression (95% decrease, P < 0.01) at 7 d. These results suggest that irradiation did not induce the expression of a Th1 pattern of cytokines. In addition, the low IL-2 expression, the other Th1 cytokine representing one of the major growth factors for the expansion of activated T cells, observed 7 d after irradiation supports this hypothesis. Although CAPE did not significantly restore the radiation-induced decrease in IL-12Rβ2 expression, IFN-γ and IL-23 expression tended to return to normality and the IL-2 mRNA level was 1.5-fold increased as compared to controls.
To address the induction mechanism of Th2 dominance by irradiation, we examined the IL-4 and IL-13 mRNA levels. As shown in Figure 1, irradiation only repressed IL-4 expression (-75%) soon after irradiation (6 h) but had no significant effect at 7 d on either IL-4 or IL-13 expression. CAPE did not influence IL-4 and IL-13 mRNA levels.
The presence of CD4+ T cells in the lamina propria was confirmed by immunostaining. The CD4+ T cell numbers were not modified at 6 h and 7 d post- irradiation compared to the control (Figure 1). CAPE did not influence the cell frequency.
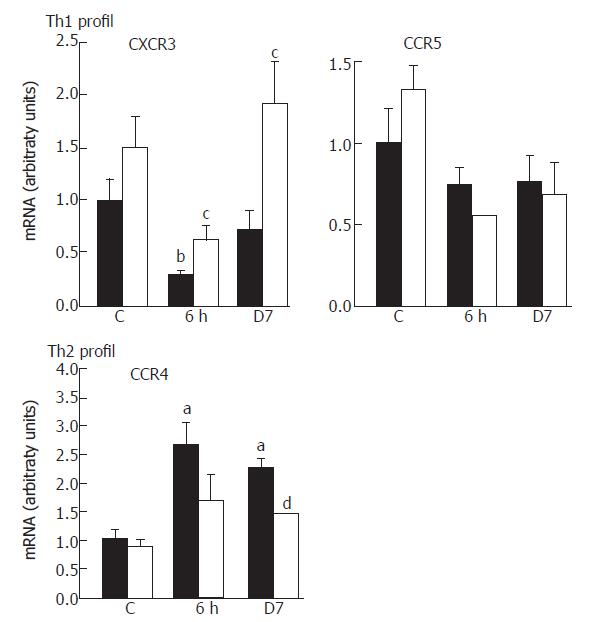
To characterize the Th cell polarization, we exploited the fact that activated Th cells acquire and maintain at high levels a specific pattern of chemokine receptors: CXCR3/CCR5 present on Th1 or CCR4 on Th2. Irradiation modified the chemokine receptor profile. There was a 70% fall (P < 0.01) in CXCR3 levels at 6 h post-irradiation and a tendency for decline in CCR5, whereas CCR4 mRNA was about 2.5-fold (P < 0.05) up-regulated at both time-points after irradiation (Figure 2). CAPE significantly counter-balanced the CXCR3 mRNA repression (P < 0.05) and the CCR4 mRNA induction (P < 0.01) at d 7.
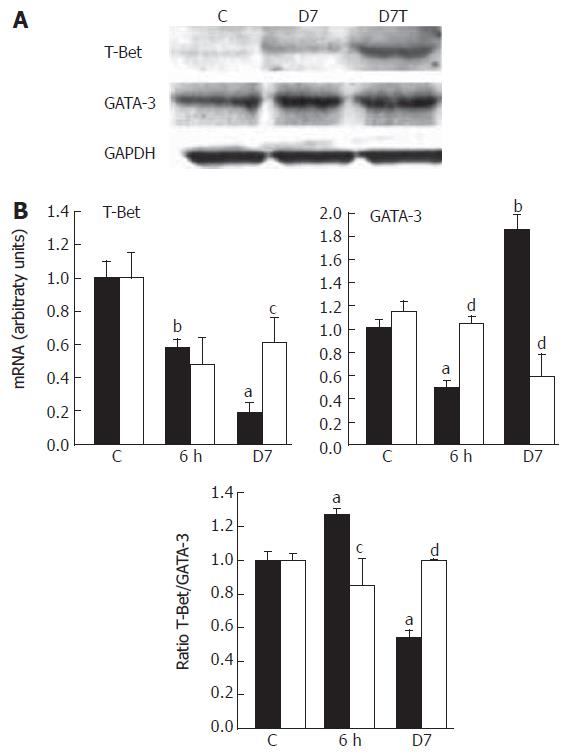
The transcription factor T-Bet is selectively expressed in Th1 cells whereas GATA-3 is selectively expressed in Th2 cells. Expression of these genes is rapidly induced in primary T cells triggering the Th1 or Th2 pathway[23]. Our data showed that irradiation induced an early (6 h) significant decrease of T-Bet expression, followed by an accentuation of decline down to 20% of the control level at d 7 (P < 0.005). In contrast, GATA-3 was significantly up-regulated at 7 d (P < 0.01, Figure 3). Because T-Bet is reported to represent Th1 immune responses and GATA-3 those of Th2, it has been suggested that the ratio of expression of these transcription factor could be used to reflect the Th1 or Th2 status in mixed cell populations[24]. The increase in GATA-3 expression was greater than for T-Bet, which was reflected by the T-Bet/GATA-3 ratio with a 2-fold decrease at d 7 (P < 0.01). These data confirm that irradiation causes a shift toward Th2 polarization. CAPE significantly reduced (P < 0.05) the radiation-induced T-Bet repression and GATA-3 was totally down-regulated at d 7 normalizing the T-bet/GATA-3 ratio. Western blot analysis confirmed the increased T-Bet/GATA-3 ratio where the T-Bet protein level reduction detected 7 d post-irradiation was removed after CAPE treatment (Figure 3).
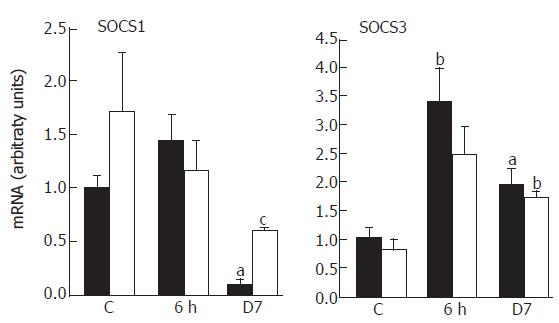
Endogenous feedback regulators of cytokine activities such as SOCS proteins regulate the amplitude and duration of the polarization influenced by T-Bet or GATA-3[10]. Since Th1 cells predominantly express SOCS1 while Th2 express high levels of SOCS3, we have hypothesized that SOCS genes might contribute to the establishment of a stable Th1 or Th2 phenotype. The analysis of the SOCS expression revealed a drastic reduction of SOCS1 mRNAs at d 7, whereas SOCS3 mRNAs were significantly up-regulated at 6 h with a high level maintained (2-fold, P < 0.05) for one week (Figure 4). The functional consequences of a high level of SOCS3 expression and a relatively low abundance of SOCS1 may drive the majority of CD4+ toward a Th2 pattern of cytokine expression. CAPE limited the irradiation effect on SOCS1, with a restoration to half the level of that of the controls at 7 d (P < 0.05), but had no effect on SOCS3 expression.
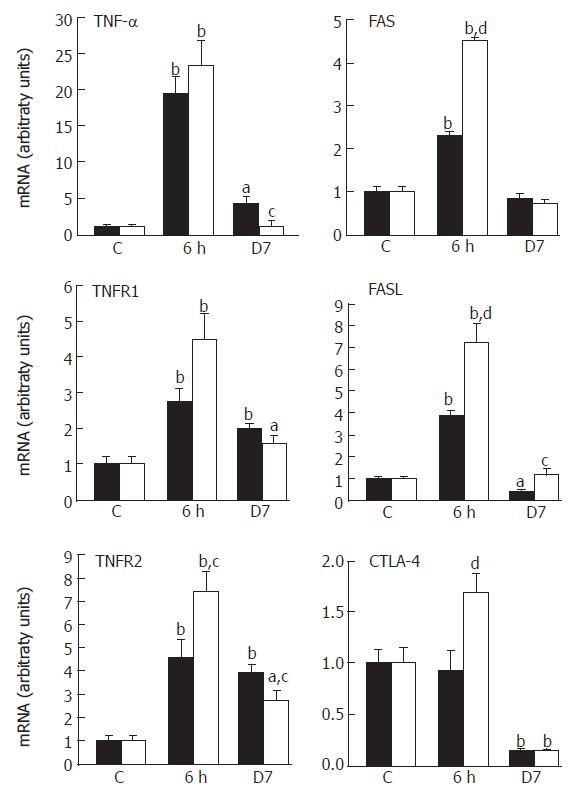
Since the decision as to whether T cells survive or undergo apoptosis is of particular importance for adaptive immune responses[14], we examined the expression of genes associated with the regulation of apoptosis, in the mucosa after irradiation. Real-time PCR was used to quantify mRNA levels of extrinsic factors (TNF-α, FasL, CTLA-4), specific receptors (TNFRs, Fas) and intrinsic mediators (XIAP, Bax, caspase-3). Irradiation led to an over-expression of TNF-α (20-fold at 6 h, P < 0.001), which correlated with a significant increase in TNF receptor (R1, R2) mRNAs (Figure 5). These over-expressions remained significant at d 7. It has been demonstrated that CAPE has anti-inflammatory and apoptotic properties[3,17]. Accordingly, CAPE significantly reduced TNF-α up-regulation at 7 d post-irradiation and led to a dual effect on TNFR2 expression with an early (6 h) amplification of expression and, in contrast, a reduction of the irradiation effect at 7 d.
The engagement of the death receptor Fas by FasL plays an important role in apoptotic events. Our data have shown that irradiation has a biphasic effect with a significant increase of Fas and FasL mRNA levels at 6 h and a significant FasL repression at d 7 (P < 0.05). CAPE over-expressed Fas/FasL at 6 h with a persistent effect on FasL mRNA level at d 7 (Figure 5). In association with Fas/FasL, CTLA-4 engagement could potentiate the elimination of pathogenic T cells[24]. Irradiation significantly down-regulated CTLA-4 at d 7 (90% decrease compared to controls, P < 0.01), which was not modified by CAPE (Figure 5). These results suggest that an absence of cytotoxic mediators occurs at 7 d post-irradiation that is minimized by CAPE-induced FasL normalization.
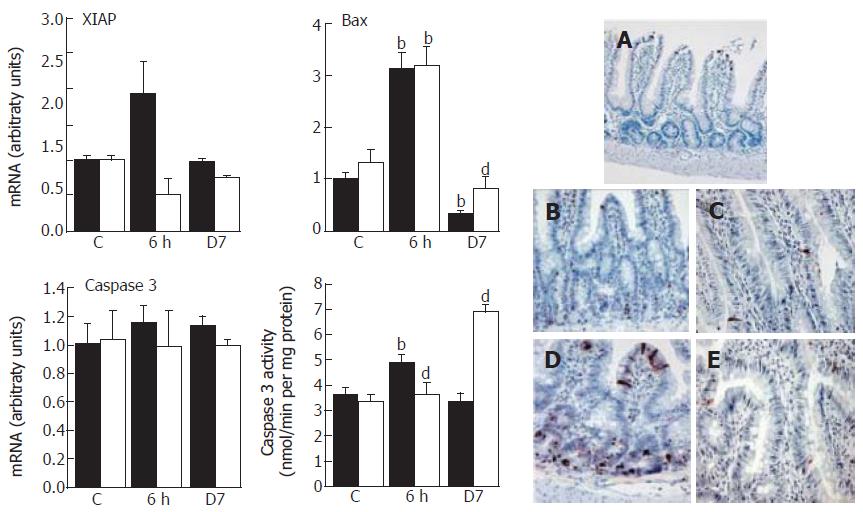
The modulation of the members of the IAP (apoptosis inhibitors) family, such as the anti-apoptotic protein XIAP or the pro-apoptotic protein Bax, regulates apoptosis. No significant change in XIAP mRNA was observed at 6 h whereas Bax expression rose at 6 h then progressively decreased at 7 d leading to a 70% reduction (P < 0.01) (Figure 6). The apoptotic event was confirmed at 6 h by an increase in caspase-3 activity that contrasted with the normal activity found at 7 d; caspase-3 expression remained invariant. CAPE reduced the XIAP expression and over-expressed Bax as compared to irradiated rats at 7 d (P < 0.01), resulting in a significant caspase-3 activity increase (P < 0.01).
The irradiation-induced apoptosis events at 6 h were confirmed by TUNEL assay (Figure 6A-E). TUNEL+ cells were sparse in the lamina propria of controls, and were only located at the top of the villi. Six hours post-irradiation, the number of apoptotic cells was considerably increased and localized in the crypts and the bottom of the villi compared to controls. Some apoptotic cells appeared in the lamina propria. The irradiation-induced apoptotic effect was amplified in the crypts and the bottom by CAPE at 6 h but not at d 7, as compared to controls.
In this study, we investigated whether targeting defective mucosal cell death underlies the sustained therapeutic benefit of CAPE by orienting the immune response induced by irradiation. The results show firstly that irradiation led to an earlier apoptotic process, which progressively resulted in an apoptotic resistance and secondly, that irradiation oriented the immune response to a predominance of Th2 type polarization. Overall, the emerging picture is that, in the non-inflamed gut, the T cells manifest increased spontaneous apoptosis mediated by the Fas pathway that limited expansion of T cells. By contrast, in the inflamed tissue, resident T cells are relatively resistant to apoptosis. Thus, they exhibit an enhanced cytokine production because of prolonged survival, which significantly aggravates the inflammation. The functional importance of T cell apoptosis resistance has been emphasized by recent studies in animal models of IBD showing that IL-12 and TNF-α antibodies appear to suppress chronic intestinal inflammation by the induction of T cell apoptosis[25]. The regulation of T cell activation by immunosuppressive drugs is known to be relevant in the treatment of inflammatory disorders. Medical or accidental exposure to radiation leads to an inflammatory process[2]. Currently, the purpose of using NF-κB inhibitors as an adjuvant for cancer treatment is to increase the therapeutic index of radiotherapy and chemotherapy[26,27]. The success of this approach relies both on its ability to promote cancerous cell killing by irradiation and to spare normal cells from enhanced damage. By analysing the immunomodulatory and apoptotic properties[17], we have shown that CAPE shifts from the radiation-induced Th2-like pattern of gene expression toward Th1 gene expression by an apoptotic sensitization.
Irradiation led to the repression of genes involved in 4 key features of Th1 cell differentiation: genes involved in Th1 differentiation (IL-12, IL-12Rβ2), genes associated with Th1 cytokine responses (IFN-γ, IL-2), genes regulating cell death and apoptosis (Fas/FasL, CTLA-4) and genes controlling Th1 migratory behaviour (CXCR3, CCR5). Simultaneous to the down-regulation of Th1-associated chemokine receptors, the transcription factor T-Bet was moderately expressed while GATA-3 was up-regulated. Transcription factors such as T-Bet and GATA-3 play a critical role in the initiation of primary immune responses and skewing the response toward the Th1 or Th2 pathway[23]. The duration of the response depends on the coordination of lymphocyte responses. These aspects of the adaptive immune response are regulated by endogenous feedback regulators of cytokine activities[10]. Thus, SOCS proteins are differentially expressed: Th1 cells predominantly express SOCS1 whereas Th2 cells express very high levels of SOCS3. The restricted pattern of expression in Th1 and Th2 cells underscores the importance of SOCS members in Th cell subtypes. A preferential up-regulation of SOCS1 expression in Th1 but not Th2 after IL-12 stimulation argues for a role of cytokine signalling in SOCS regulation[10,22]. In our study, we found a differential expression of SOCS at 7 d post-irradiation with a repression of SOCS1 and an overexpression of SOCS3. The functional consequence of SOCS3 expression may be to drive the majority of CD4+ toward a Th2 pattern of cytokine expression at this time. However, cytokines can override the effects of SOCS3 during Th differentiation. In fact, a major effect of IL-12 signalling in non-polarized Th cells is to down-regulate SOCS3 expression in cells committed to the Th1 lineage. In this way, Th1 differentiation requires IL-12/IL-12Rβ2 signalling[28] and SOCS1[24], and the drastic repression of IL-23, closely related to IL-12, that occurred at 7 d post-irradiation reduces the possibility of a Th1 lineage. In addition, IL-12/IL-12Rβ2 signalling supports high IFN-γ production and IFN signalling to down-regulate IL-4 and prevent Th2 dominance[29]. In this way, although IL-4 expression induced by irradiation was not elevated, the protection of Th1 dominance after irradiation could not maintained by the mere fact of the IFN-γ repression at d 7. Szabo et al[7] have shown that when IFN-γ production is limited, a low IL-4 production in early responses may critically inhibit IL-12Rβ2 expression with a significant effect on the development of a Th1/Th2 shift. In fact, only low IL-4 level is required in the complete absence of extrinsic Th1 signals (e.g: IL-12) to generate Th2 CD4+. These results showed the same prevalence of an irradiation-induced Th2 response in the lung. In fact, enhanced lymphocyte reactivity, dominated by Th2 cells, has been shown in radiation-induced pneumonitis and subsequent pulmonary fibrosis, suggesting a critical role for Th2 CD4+[30].
TUNEL staining has revealed an increase of apoptotic cells observed at the top of the villi and in the crypts. Some apoptotic cells appeared after irradiation in the lamina propria which is formed from a majority of immune cells. In fact, a differential sensitivity of lymphocyte subpopulations to irradiation-induced apoptosis has been reported; in particular, CD4+ T cells are relatively radioresistant[31]. In this work, CD4+ immunostaining confirmed their presence after irradiation. TUNEL positive cells increased at 6 h confirming the radiosensitization effect of CAPE but without real modification of the number of CD4+ cells. In this study and in accordance with other findings[27], the earliest intestinal response to radiation injury reflects a complex series of apoptotic events involving extrinsic factors (TNF-α, FasL, IL-2), specific receptors (TNFR, Fas) and intrinsic mediators (XIAP, Bax and caspase-3). All these apoptotic events occurred rapidly after irradiation (6 h) but were amply repressed one week post-irradiation.
The extrinsic factors such as Fas and FasL are particu-larly important in T cell apoptotic events. The observed decrease in Fas/FasL expression may mediate T cells apoptosis and becomes especially important in the irradiation-inflammatory process at d 7, because failures of this mortality pathway can impair both autocrine and paracrine mechanisms for eliminating activated T cells. In fact, Fas is expressed by the both Th cell subsets whereas only Th1 cells express detectable amounts of FasL[29]. The differential expression of FasL by Th1 and Th2 cells is correlated with the ability of these cells to undergo to apoptosis: Th1 clones are highly sensitive to apoptosis, whereas Th2 clones are relatively resistant. In this way, the genesis of Th2 orientation may occur early (6 h) post-irradiation. In view of the ability of CAPE to induce an apoptotic event via Fas activation[32], the restoration of FasL expression by CAPE may suggest a modification of the apoptosis resistance and of the Th1/Th2 balance at 7 d post-irradiation. This hypothesis must be confirmed by in vitro experiments. Interestingly in respect to asthma, it has been shown that intratracheal delivery of adenovirus encoding FasL could significantly suppress pulmonary Th2 immune response[33].
Fas, a member of the TNF receptor family, is inducible by TNF-α itself[34], a good correlation was observed concerning the radiation-induced expression variation between Fas/FasL and TNF-α. CAPE did not alter this high mRNA level at 6 h, but this correlation disappeared at 7 d with a repression of TNF-α and a normalization of FasL levels. The cell death signal induced by TNF-α is mediated by TNFR1. In contrast, the engagement of TNFR2 promotes cell survival by the induction of anti-apoptotic molecules[35]. Although TNF-α was repressed at 7 d, the TNFR1 up-regulation induced by irradiation was not altered by CAPE in contrast to TNFR2 which was down-regulated. This observation, associated with FasL normalization, may be an indication of a limitation of apoptosis resistance induced by CAPE at 7 d. Intrinsic gene product analysis confirmed this argument at this time when CAPE also normalized Bax expression, contributing to increased caspase-3 activity.
Cell death is a complex series of events regulated by the intrinsic propensity of individual cells, the environment and cell surface. The regulation of T-cell activation/apoptosis depends on critical cell surface molecules, in particular CTLA-4 which down-regulates T cell activation. The immune importance of CTLA-4 has been reported in vivo where CTLA-4 blockade exacerbates some autoimmune diseases[36] and CTLA-4-deficient mice exhibit a massive polyclonal increase in T cells. On the basis of these properties and in view of our data which showed that CTLA-4 was totally repressed at 7 d, it seems possible that an irradiation-induced T-cell activation effect may have occurred. CAPE had no effect on CTLA-4 expression, so that it seems probable that the T cell expansion/activation was maintained, but an orientation of the immune response cannot be excluded.
These results suggest that CAPE, by modulating the cytokine pattern, changed the orientation of immune responses after irradiation and favoured a Th1 response notably by the total or partial suppression of IL-12 and IFN-γ repression. Interestingly, CAPE could inhibit T-cell activation by targeting both NF-κB and the nuclear factor of activated T cells (NFAT)[37]. Diehl et al[38] showed that NFAT activation in CD4+ is required for the development of Th2 differentiation and its inhibition in T cells prevents the Th2 immune response in a model of allergic pulmonary inflammation. The activation of NFAT by irradiation still has not been demonstrated but we cannot exclude its potential involvement in the irradiation-induced Th2-like profile.
S- Editor Pan BR L- Editor Lalor PF E- Editor Bi L
| 1. | Andreyev J. Gastrointestinal complications of pelvic radiotherapy: are they of any importance. Gut. 2005;54:1051-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 2. | Linard C, Ropenga A, Vozenin-Brotons MC, Chapel A, Mathe D. Abdominal irradiation increases inflammatory cytokine expression and activates NF-kappaB in rat ileal muscularis layer. Am J Physiol Gastrointest Liver Physiol. 2003;285:G556-G565. [PubMed] |
| 3. | MacDonald TT, Monteleone G, Pender SL. Recent developments in the immunology of inflammatory bowel disease. Scand J Immunol. 2000;51:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 157] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Cêtre C, Pierrot C, Cocude C, Lafitte S, Capron A, Capron M, Khalife J. Profiles of Th1 and Th2 cytokines after primary and secondary infection by Schistosoma mansoni in the semipermissive rat host. Infect Immun. 1999;67:2713-2719. [PubMed] |
| 5. | Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1152] [Cited by in RCA: 1124] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 6. | Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1030] [Cited by in RCA: 1114] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 7. | Heath VL, Showe L, Crain C, Barrat FJ, Trinchieri G, O'Garra A. Cutting edge: ectopic expression of the IL-12 receptor-beta 2 in developing and committed Th2 cells does not affect the production of IL-4 or induce the production of IFN-gamma. J Immunol. 2000;164:2861-2865. [PubMed] |
| 8. | Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 907] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 9. | Ritz SA, Cundall MJ, Gajewska BU, Swirski FK, Wiley RE, Alvarez D, Coyle AJ, Stampfli MR, Jordana M. The lung cytokine microenvironment influences molecular events in the lymph nodes during Th1 and Th2 respiratory mucosal sensitization to antigen in vivo. Clin Exp Immunol. 2004;138:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Egwuagu CE, Yu CR, Zhang M, Mahdi RM, Kim SJ, Gery I. Suppressors of cytokine signaling proteins are differentially expressed in Th1 and Th2 cells: implications for Th cell lineage commitment and maintenance. J Immunol. 2002;168:3181-3187. [PubMed] |
| 11. | Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1493] [Cited by in RCA: 1485] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 12. | Rathmell JC, Thompson CB. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell. 2002;109 Suppl:S97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 328] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 13. | Xiao C, Ghosh S. NF-kappaB, an evolutionarily conserved mediator of immune and inflammatory responses. Adv Exp Med Biol. 2005;560:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | Perkins ND. The Rel/NF-kappa B family: friend and foe. Trends Biochem Sci. 2000;25:434-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 277] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 15. | Murphy FJ, Hayes I, Cotter TG. Targeting inflammatory diseases via apoptotic mechanisms. Curr Opin Pharmacol. 2003;3:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Levine AD, Fiocchi C. Regulation of life and death in lamina propria T cells. Semin Immunol. 2001;13:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Orban Z, Mitsiades N, Burke TR Jr, Tsokos M, Chrousos GP. Caffeic acid phenethyl ester induces leukocyte apoptosis, modulates nuclear factor-kappa B and suppresses acute inflammation. Neuroimmunomodulation. 2000;7:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Fitzpatrick LR, Wang J, Le T. Caffeic acid phenethyl ester, an inhibitor of nuclear factor-kappaB, attenuates bacterial peptidoglycan polysaccharide-induced colitis in rats. J Pharmacol Exp Ther. 2001;299:915-920. [PubMed] |
| 19. | Brink N, Szamel M, Young AR, Wittern KP, Bergemann J. Comparative quantification of IL-1beta, IL-10, IL-10r, TNFalpha and IL-7 mRNA levels in UV-irradiated human skin in vivo. Inflamm Res. 2000;49:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 734] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 21. | Becker C, Wirtz S, Blessing M, Pirhonen J, Strand D, Bechthold O, Frick J, Galle PR, Autenrieth I, Neurath MF. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J Clin Invest. 2003;112:693-706. [PubMed] |
| 22. | Eyles JL, Metcalf D, Grusby MJ, Hilton DJ, Starr R. Negative regulation of interleukin-12 signaling by suppressor of cytokine signaling-1. J Biol Chem. 2002;277:43735-43740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Chakir H, Wang H, Lefebvre DE, Webb J, Scott FW. T-bet/GATA-3 ratio as a measure of the Th1/Th2 cytokine profile in mixed cell populations: predominant role of GATA-3. J Immunol Methods. 2003;278:157-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Campanelli AP, Martins GA, Souto JT, Pereira MS, Livonesi MC, Martinez R, Silva JS. Fas-Fas ligand (CD95-CD95L) and cytotoxic T lymphocyte antigen-4 engagement mediate T cell unresponsiveness in patients with paracoccidioidomycosis. J Infect Dis. 2003;187:1496-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Neurath MF, Finotto S, Fuss I, Boirivant M, Galle PR, Strober W. Regulation of T-cell apoptosis in inflammatory bowel disease: to die or not to die, that is the mucosal question. Trends Immunol. 2001;22:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 129] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Lin A, Karin M. NF-kappaB in cancer: a marked target. Semin Cancer Biol. 2003;13:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 281] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 27. | Wang Y, Meng A, Lang H, Brown SA, Konopa JL, Kindy MS, Schmiedt RA, Thompson JS, Zhou D. Activation of nuclear factor kappaB In vivo selectively protects the murine small intestine against ionizing radiation-induced damage. Cancer Res. 2004;64:6240-6246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Sakamoto S, Fukushima A, Ozaki A, Ueno H, Kamakura M, Taniguchi T. Mechanism for maintenance of dominant T helper 1 immune responses in Lewis rats. Microbiol Immunol. 2001;45:373-381. [PubMed] |
| 29. | Roberts AI, Devadas S, Zhang X, Zhang L, Keegan A, Greeneltch K, Solomon J, Wei L, Das J, Sun E. The role of activation-induced cell death in the differentiation of T-helper-cell subsets. Immunol Res. 2003;28:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Westermann W, Schöbl R, Rieber EP, Frank KH. Th2 cells as effectors in postirradiation pulmonary damage preceding fibrosis in the rat. Int J Radiat Biol. 1999;75:629-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Seki H, Iwai K, Kanegane H, Konno A, Ohta K, Ohta K, Yachie A, Taniguchi N, Miyawaki T. Differential protective action of cytokines on radiation-induced apoptosis of peripheral lymphocyte subpopulations. Cell Immunol. 1995;163:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Watabe M, Hishikawa K, Takayanagi A, Shimizu N, Nakaki T. Caffeic acid phenethyl ester induces apoptosis by inhibition of NFkappaB and activation of Fas in human breast cancer MCF-7 cells. J Biol Chem. 2004;279:6017-6026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 170] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 33. | Chuang YH, Fu CL, Lo YC, Chiang BL. Adenovirus expressing Fas ligand gene decreases airway hyper-responsiveness and eosinophilia in a murine model of asthma. Gene Ther. 2004;11:1497-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Choi C, Park JY, Lee J, Lim JH, Shin EC, Ahn YS, Kim CH, Kim SJ, Kim JD, Choi IS. Fas ligand and Fas are expressed constitutively in human astrocytes and the expression increases with IL-1, IL-6, TNF-alpha, or IFN-gamma. J Immunol. 1999;162:1889-1895. [PubMed] |
| 35. | Kaiser GC, Polk DB. Tumor necrosis factor alpha regulates proliferation in a mouse intestinal cell line. Gastroenterology. 1997;112:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. | Eagar TN, Karandikar NJ, Bluestone JA, Miller SD. The role of CTLA-4 in induction and maintenance of peripheral T cell tolerance. Eur J Immunol. 2002;32:972-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Márquez N, Sancho R, Macho A, Calzado MA, Fiebich BL, Muñoz E. Caffeic acid phenethyl ester inhibits T-cell activation by targeting both nuclear factor of activated T-cells and NF-kappaB transcription factors. J Pharmacol Exp Ther. 2004;308:993-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |