Published online Aug 14, 2006. doi: 10.3748/wjg.v12.i30.4922
Revised: April 14, 2006
Accepted: April 24, 2006
Published online: August 14, 2006
A 77-year-old man on systemic chemotherapy against postoperative bilateral multiple lung metastases of malignant solitary fibrous tumor of the pleura suffered from pruritus and jaundice. Blood examination showed elevated levels of hepatobiliary enzymes. Abdominal computed tomography showed a tumor with peripheral enhancement in the pancreatic head, accompanied with the dilatation of intra- and extra-hepatic bile ducts. He was diagnosed as having obstructive jaundice caused by a pancreatic head tumor. The pancreatic head tumor was presumably diagnosed as the metastasis of malignant solitary fibrous tumor of the pleura, because the findings on the pancreatic head tumor on abdominal CT were similar to those on the primary lung lesion of malignant solitary fibrous tumor of the pleura. The pancreatic tumor grew rapidly after the implantation of metallic stent in the inferior part of the common bile duct. The patient died of lymphangitis carcinomatosa of the lungs. Autopsy revealed a tumor that spread from the pancreatic head to the hepatic hilum. Microscopically, spindle-shaped cells exhibiting nuclear atypicality or division together with collagen deposition were observed. Immunohistochemically the pancreatic head tumor cells were negative for staining of α-smooth muscle actin (α-SMA) or CD117, but positive for vimentin, CD34 and CD99. These findings are consistent with those on malignant solitary fibrous tumor of the pleura. We report the first case of obstructive jaundice caused by a secondary pancreatic tumor from malignant solitary fibrous tumor of the pleura.
- Citation: Yamada N, Okuse C, Nomoto M, Orita M, Katakura Y, Ishii T, Shinmyo T, Osada H, Maeda I, Yotsuyanagi H, Suzuki M, Itoh F. Obstructive jaundice caused by secondary pancreatic tumor from malignant solitary fibrous tumor of pleura: A case report. World J Gastroenterol 2006; 12(30): 4922-4926
- URL: https://www.wjgnet.com/1007-9327/full/v12/i30/4922.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i30.4922
Solitary fibrous tumor of the pleura (SFTP) is a neoplasm derived from mesenchymal cells located in the submesothelial lining of the pleural space, predominantly composed of spindle-shaped cells in combination with collagen deposition[1]. SFTP comprises approximately 5% of primary pleural tumors following malignant mesothelioma[2]. Seven percent to 13% of SFTPs are considered to be malignant neoplasms, of which 41% to 63% recur in the pleura or lung or metastasize to the liver, brain, spleen, adrenal gland and other organs[1,2]. However, no metastasis to the pancreatic head with obstructive jaundice has been reported. Here, we report the first case of obstructive jaundice caused by a secondary pancreatic tumor from malignant SFTP.
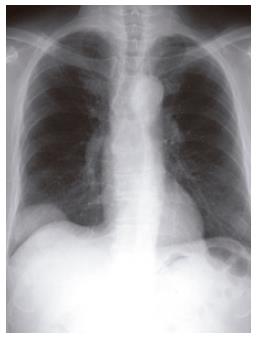
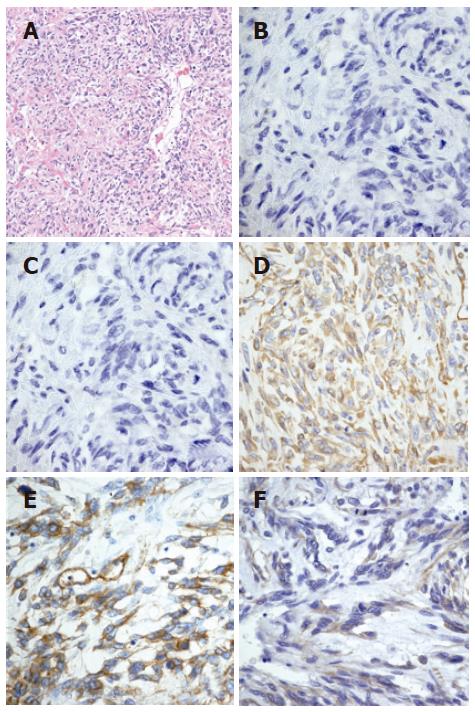
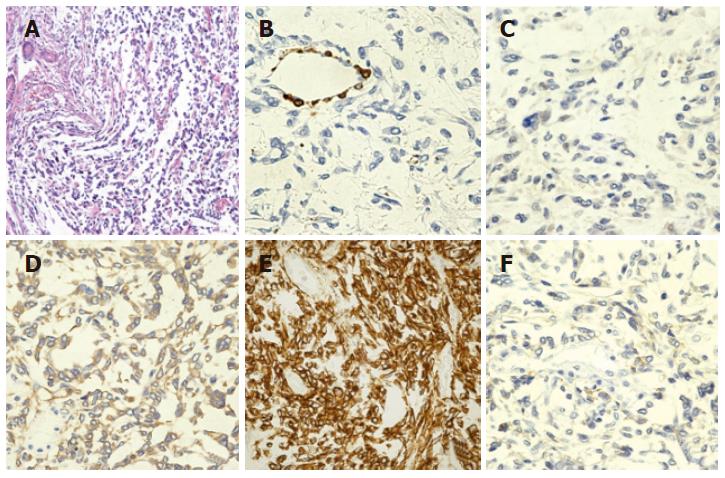
The patient was a 77-year-old Japanese man. In July 2001, he was found to have an extrapleural tumor projecting from the right diaphragm by mass screening chest roentgenography. Partial resections of the right diaphragm and lower right lobe of the lungs were carried out in July 2002, because of the rapid tumor growth (Figure 1). The tumor in the resected specimens was composed of nonorganized, spindle-shaped cells exhibiting nuclear atypicality or division together with collagen deposition. Immunohistochemical staining showed that the tumor cells were negative for α-SMA or CD117 (c-Kit) but positive for vimentin, CD34 and CD99 (Figures 2A-F). He was diagnosed as having a solitary fibrous tumor of the pleura (SFTP).
In September 2003, multiple metastases were found in the bilateral lung fields and he received systemic chemotherapy with gemcitabine (GEM) and cisplatin (CDDP). Two courses of chemotherapy with GEM and CDDP temporarily reduced the tumor size in the lung.
In mid-April 2004, he experienced pruritus and showed skin jaundice. He was found to have liver dysfunction. He was admitted to our hospital for further evaluation and treatment.
His medical history before 2001 was uneventful. He had a history of daily alcohol intake for 50 years and daily smoking for 40 years.
On the day of admission, he was alert. His body temperature was 35.4°C. His bulbar conjunctiva and skin were icteric. There was a surgical scar on his right lateral thorax. His liver was soft and palpable 3 cm below the umbilical margin. No tumor was palpated in the abdomen. A moveable and elastic tumor 2 cm in diameter was palpable in his right buttock.
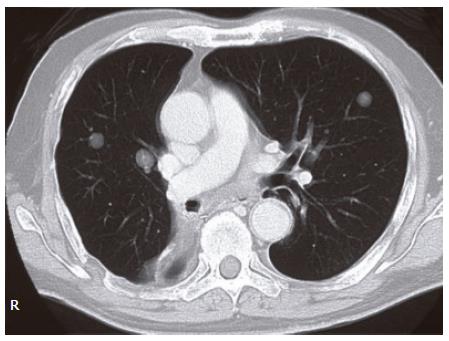
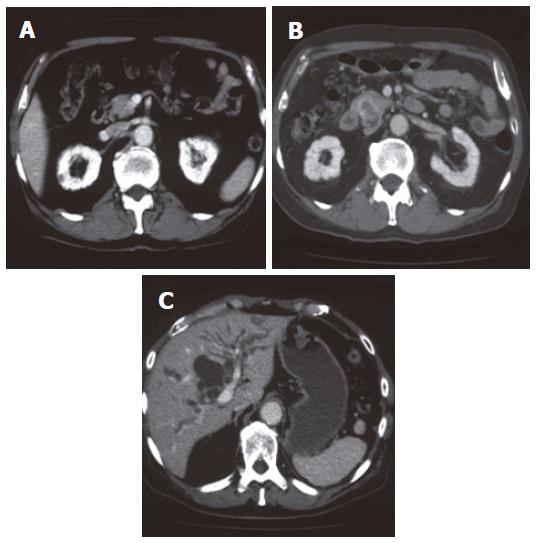
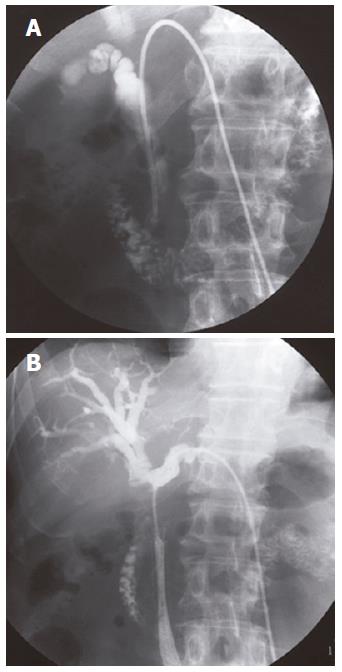
The levels of hepatobiliary enzymes and bilirubin were elevated. The levels of tumor markers including carcinoembryonic antigen (CEA) and pancreatic cancer-associated antigen-2 (DUPAN-2) were normal (Table 1). Multiple nodular lesions were observed in the bilateral lung fields by chest computed tomography (CT) (Figure 3). By abdominal CT, a tumor (25 mm × 17 mm) with its periphery enhanced by the contrast material in the delayed phase, was observed in the pancreatic head (Figure 4B). This tumor was not detected by preoperative abdominal CT in 2002 (Figure 4A). The dilatation of intra- and extra-hepatic bile ducts was also observed. Intra-abdominal lymph nodes were not swollen (Figure 4C). Because the radiological findings observed with or without contrast material were similar to those observed in the primary pleural tumor, he was diagnosed as having obstructive jaundice presumably caused by a secondary pancreatic head tumor from malignant SFTP. Percutaneous transhepatic bile duct drainage (PTBD) was performed to improve his jaundice. Cholangiography via a PTBD tube showed marked stenosis of the inferior part of the common bile duct (CBD) (Figure 5A). No atypical cell was observed in the bile collected by the tube.
| Peripheral blood | TP | 6.3 mg/dL | |
| WBC | 4900 /μL | Alb | 3.8 mg/dL |
| Hb | 13.8 g/dL | Cr | 0.96 mg/dL |
| Hct | 41.2% | BUN | 13.4 mg/dL |
| Plt | 16.5 × 104 μL | Amy | 107 IU/L |
| Coagulation | FBS | 111 mg/dL | |
| PT | 100% | CRP | 0.08 mg/dL |
| APTT | 28.5 (cont: 29.3) | Tumor Markers | |
| Fib | 499 mg/dL | CA 19-9 | 16 U/mL |
| Blood Chemistry | CEA | 4.2 ng/mL | |
| T-Bil | 4.2 mg/dL | DUPAN-2 | 135 U/mL |
| D-Bil | 3.4 mg/dL | SCC | 1.2 ng/mL |
| AST | 318 IU/L | NSE | 6.6 ng/mL |
| ALT | 444 IU/L | ||
| LDH | 405 IU/L | ||
| ALP | 1860 IU/L | ||
| γ-GTP | 612 IU/l |
Because of the bilateral multiple lung and right-buttock tumors, which were considered to have metastasized from the pleura, pancreatoduodenectomy was no longer an indication for treatment.
Thus, a metallic stent was implanted in the inferior part of CBD as a palliative treatment. The levels of hepatobiliary enzymes became normal after the implantation. However, in July 2004, he suffered from fever and jaundice with elevated levels of hepatobiliary enzymes. Abdominal CT demonstrated obstruction of the stent and dilataion of the intrahepatic bile ducts. Furthermore, an obstruction from the inferior part of CBD to the confluence of the right and left extra-hepatic bile ducts involving the whole stent was observed when cholangiography was performed again (Figure 5B). Although a drainage tube was reinserted in the anterior and lateral branches of the intra-hepatic bile ducts, he developed lymphangitis carcinomatosa and died of respiratory failure.
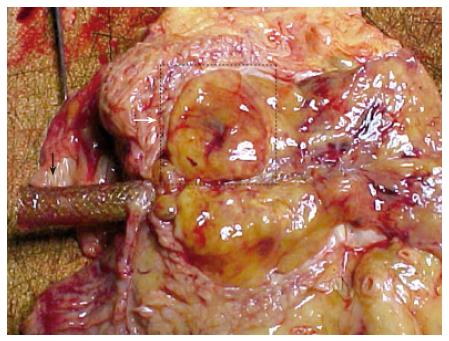
Autopsy was performed with informed consent. Multiple tumors were observed in the bilateral lung. A solitary tumor (25 mm × 15 mm × 15 mm) was observed in his right buttock. A large tumor (120 mm × 30 mm × 20 mm) spread from the pancreatic head to the hepatic hilum and involved bilateral hepatic ducts (Figure 6).
Microscopically, spindle-shaped cells exhibiting nuclear atypicality or division together with collagen deposition were observed (Figure 7A). Immunohistochemically, tumor cells of the pancreatic head were negative for α-SMA or CD117, but positive for vimentin, CD34 and CD99 (Figures 7B-F). These findings were consistent with those on malignant SFTP resected in July 2002.
Malignant SFTP recurs in the pleura or lung or metastasizes to the liver, brain, spleen, adrenal gland and other organs via a blood-borne pathway[1-3]. This present patient was considered to have a pancreatic head tumor having metastasized from a malignant SFTP for the following reasons. Multiple metastatic lesions in the bilateral lung and a growing subcutaneous tumor were observed and the findings on the pancreatic head tumor by abdominal CT were similar to those on the primary lung SFTP. The tentative diagnosis was confirmed by the histopathological and immunohistochemical findings on autopsy.
Secondary pancreatic tumor is rare. Nakamura et al[4] reported that the incidence of secondary pancreatic tumor is approximately 6% in autopsy studies and increases to approximately 15% in cases of malignant tumors. The lung is the second most common primary site (18%) of secondary pancreatic tumor[4,5]. The histopathological types of this tumor are large cell carcinoma, small cell carcinoma, adenocarcinoma and squamous cell carcinoma[4-7]. Malignant SFTP has never been reported as a cause of secondary pancreatic tumor to date.
In general, secondary pancreatic tumor is caused by blood-borne metastasis, lymphatic metastasis and tumor infiltration from adjacent organs or peritoneal seeding. The secondary pancreatic tumor of this present patient was considered to be caused by blood-borne metastasis because no metastasis in regional lymph nodes was observed during operation or on autopsy, but multiple metastatic lesions in the bilateral lungs and a subcutaneous metastatic tumor in the right buttock were observed.
Although radiation therapy and chemotherapy have been performed against malignant SFTP in addition to resection, they could not achieve sufficient therapeutic efficacy[2]. On the other hand, GEM monotherapy or combination therapy with CDDP is effective against malignant mesothelioma[8].
Chemotherapy with GEM and CDDP, which is effective for patients with malignant mesothelioma, was selected for this patient because the treatment of malignant SFTP with metastasis was not authorized, and both malignant mesothelioma and SFTP originated from the pleura. However, the tumor grew more rapidly even after chemotherapy. Forty-one percent to 63% of malignant SFTPs recur locally or metastasize to other organs[1,2] and 23% of these patients die within 24 mo of diagnosis[9]. Therefore, the establishment of an effective anti-tumor therapy against malignant SFTP is desired.
The leading drug for the novel therapy is imatinib mesylate, which is effective against gastrointestinal stromal tumors (GISTs)[10]. In half of the patients with malignant SFTP, tumor cells have been found positive for c-kit[11]. Therefore, imatinib mesylate, which inhibits kit-thyrosine kinase, may be a promising drug for molecular-target-based therapy. This hypothesis should be verified in future studies.
In summary, we report a rare case of obstructive jaundice caused by a secondary pancreatic tumor from malignant SFTP. Pancreatic metastasis may adversely affect the prognosis of malignant SFTP. Therefore, abdominal images and the levels of hepatobiliary enzymes should be examined regularly.
S- Editor Wang J L- Editor Wang XL E- Editor Bai SH
| 1. | John DM. Solitary Fibrous Tumor of the Pleura. Semi Thorac Cardiovasc Surg. 2003;15:305-309. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol. 1989;13:640-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 953] [Cited by in RCA: 848] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 3. | Briselli M, Mark EJ, Dickersin GR. Solitary fibrous tumors of the pleura: eight new cases and review of 360 cases in the literature. Cancer. 1981;47:2678-2689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Nakamura E, Shimizu M, Itoh T, Manabe T. Secondary tumors of the pancreas: clinicopathological study of 103 autopsy cases of Japanese patients. Pathol Int. 2001;51:686-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Roland CF, van Heerden JA. Nonpancreatic primary tumors with metastasis to the pancreas. Surg Gynecol Obstet. 1989;168:345-347. [PubMed] |
| 6. | Johnson DH, Hainsworth JD, Greco FA. Extrahepatic biliary obstruction caused by small-cell lung cancer. Ann Intern Med. 1985;102:487-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Whittington R, Moylan DJ, Dobelbower RR, Kramer S. Pancreatic tumours in patients with previous malignancy. Clin Radiol. 1982;33:297-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Byrne MJ, Davidson JA, Musk AW, Dewar J, van Hazel G, Buck M, de Klerk NH, Robinson BW. Cisplatin and gemcitabine treatment for malignant mesothelioma: a phase II study. J Clin Oncol. 1999;17:25-30. [PubMed] |
| 9. | de Perrot M, Fischer S, Bründler MA, Sekine Y, Keshavjee S. Solitary fibrous tumors of the pleura. Ann Thorac Surg. 2002;74:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 295] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 10. | Dagher R, Cohen M, Williams G, Rothmann M, Gobburu J, Robbie G, Rahman A, Chen G, Staten A, Griebel D. Approval summary: imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin Cancer Res. 2002;8:3034-3038. [PubMed] |
| 11. | Butnor KJ, Burchette JL, Sporn TA, Hammar SP, Roggli VL. The spectrum of Kit (CD117) immunoreactivity in lung and pleural tumors: a study of 96 cases using a single-source antibody with a review of the literature. Arch Pathol Lab Med. 2004;128:538-543. [PubMed] |