Published online Aug 14, 2006. doi: 10.3748/wjg.v12.i30.4911
Revised: October 11, 2004
Accepted: December 14, 2004
Published online: August 14, 2006
We described a 59-year-old male patient who underwent liver transplantation in 1989 for hepatocellular carcinoma (HCC) complicating hepatitis B virus (HBV) cirrhosis. In 2001 (12 years after liver transplantation), he developed a lung metastasis of HCC without intrahepatic recurrence and the resection was done. In July 2003, he was symptom free without any recurrence. HCC metastasis can develop even after a very long time of liver transplantation. Many HCCs grow slowly, and the growth rate of recurrent tumors in patients receiving immunosuppressive therapy is significantly greater than that of those who do not receive immunosuppressive therapy.
- Citation: Viola C, Asselah T, Samuel D, Durand F, Boudjema H, Valla D, Marcellin P. Solitary pulmonary metastasis arising thirteen years after liver transplantation for HBV-related hepatocellular carcinoma. World J Gastroenterol 2006; 12(30): 4911-4913
- URL: https://www.wjgnet.com/1007-9327/full/v12/i30/4911.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i30.4911
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world and the third cause of cancer-related death[1]. Cirrhosis mainly caused by hepatitis B and C viruses (HBV and HBC) constitutes the main risk factor for HCC with an yearly cumulative incidence of 3%[1].
Liver transplantation is claimed to simultaneously cure the tumor and the underlying cirrhosis in selected patients. The 5-year survival rate can be achieved in 75% of optimal candidates for liver transplantation (single nodule < 5 cm or up to three nodules < 3 cm in diameter) with a recurrence rate below 15%[1,2]. For patients with a single resectable HCC complicating cirrhosis, an alternative strategy is to offer resection first and then liver transplantation, if the tumor recurs or if the liver function deteriorates (salvage OLT)[3].
However, the rate of HCC recurrence is high even after liver transplantation[4]. The most frequent sites of recurrence of HCC after OLT are the lung (with a frequency of 51%) and the liver allograft (46%)[4,5]. The great majority of cancer recurrences appear within 5 years of liver transplantation.
We described a 59-year-old male patient who underwent liver transplantation in 1989 for HCC complicating HBV cirrhosis. In 2001 (12 years after liver transplantation), he developed a lung metastasis of HCC without intrahepatic recurrence and the resection was done. In July 2003, he was symptom free without any recurrence.
A 59-year-old man developed deep fatigue and jaundice in May 1986. His serum alanine aminotransaminase was 145 IU/L (normal < 40 IU/L) and tests for serum HBsAg, HBcAb, and HbeAb were positive, HBV-DNA was undetectable by hybridization assay. A liver biopsy showed cirrhosis. Serum level of alpha-feto-protein was 200 ng/mL (normal ≤ 15 ng/mL). Imaging studies (computerized tomography and ultrasound) showed a nodule (2 cm in diameter) in segment V with typical features of HCC.
In February 1987, segment V was resected. Histological examination confirmed a 2-cm nodule of HCC with a pseudo-capsule characterized by a sclerotic tissue with an intravascular extension of the tumor. After the intervention, alpha-feto-protein decreased to 20 ng/mL.
In December 1988, alpha-feto-protein raised to 100 ng/mL. Ultrasound and arteriography revealed nodules in the segments IV and VIII. The patient received three cures of chemoembolization (from December 1988 to March 1989) and 3 MU interferon thrice a week for 3 mo until liver transplantation, in order to make serum HBV-DNA negative. In August 1989, orthotopic liver transplantation was performed. Histological examination of the implanted liver showed micronodular cirrhosis without any evidence of neoplasia. He was given cyclosporin (18 mL/d), prednisone (20 mg/d) and azathioprine (150 mg/d). During the following 2 mo (October 1989), he was treated with cyclosporin (10 mL/d), prednisone (15 mg/d), and azathioprine (50 mg/d). During the following 14 mo (October 1990), he was treated with cyclosporin (0.6 mL/d), prednisone (10 mg/d), and azathioprine (50 mg/d).
During the following years, he had a 4-mo evaluation. Transaminase, alpha-feto-protein and imaging studies (ultrasound and thoraco-abdominal CT) did not show any sign or symptom of HCC recurrence. Rejection and opportunistic infections did not occur. He did not receive HBsAb immunoglobulins and had no reactivation of HBV infection.
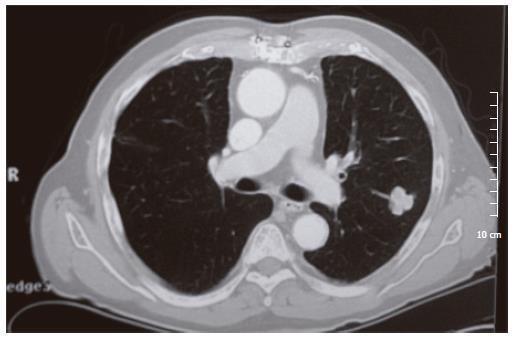
In July 2001 (143 mo after liver transplantation), CT scan revealed a 2-cm isolated nodule in the upper left lung lobe, which was polylobulated and homogeneous without calcification (Figure 1). Alpha-feto-protein increased for the first time to 105 ng/mL. He was treated with prednisone (4 mg/d) and azathioprine (50 mg/d). In May 2002 (153 mo after liver transplantation), an atypical resection of the upper left lobe was performed. Histological examination showed poorly differentiated HCC metastasis. After the intervention, alpha-feto-protein returned to its normal level (3.5 μg). Repeated biochemical and imaging examinations did not show any recurrence in the lung, liver or other organs.
At the last follow-up visit (167 mo after liver trans-plantation and 14 mo after lung resection), he was symptom free. Thoraco-abdominal CT scan did not show any recurrence (July 2003) and serum alpha-feto-protein was 2 ng/mL (normal ≤ 15 ng/mL).
Recombinant interferon-α (IFN-α) treatment prior to liver transplantation does not seem to reduce the rate of HBV infection; a residual infectivity may persist even in the absence of detectable serum HBV-DNA by standard method and its role in the carcinogenetic process cannot be eliminated[6].
The 5-year survival rate can be achieved in 75% of optimal candidates for liver transplantation (early HCC, single nodule < 5 cm or up to three nodules < 3 cm in diameter) with a recurrence-free survival rate of 92%[7]. Most recurrent tumors arise during the first 2 years after resection, which might be explained by the multicentric nature of HCC in cirrhotic livers rather than by intrahepatic metastasis. The multifocal nature of HCC was examined in the livers from patients undergoing liver transplantation. Certain histological findings in the implant, such as the presence of capsular and microvascular invasion, are considered as signs of a more aggressive tumor associated with a greater incidence of recurrence[8].
When recurrence after liver transplantation occurs, the most frequent sites are lungs (51%), liver allograft (46%), and lymph nodes (43%). Ferris et al[4] have reported a mean interval of 18 mo.
In the three major studies[4,9,10] involving HCC, the recurrence rate is 34% in 438 patients after liver transplantation (Table 1).
In a great majority of cases, recurrence of HCC occurs within 5 years of liver transplantation. The longest time to recurrence described in the literature is 124 mo[4]. To our knowledge, the time between liver transplantation and recurrence is the longest in our case (12 years, 143 mo).
An active approach to the management of resectable pulmonary metastasis from HCC is justified in selected patients, which can permit a prolonged survival[11]. Most pulmonary metastases of HCC are multiple and not amenable to surgical resection. If any solitary pulmonary metastasis encountered is resectable, the patient should undergo surgery.
The selection of patients with early HCC is the main factor affecting HCC recurrence after liver transplantation. At this early stage of tumor development, there are no other factors that have prognostic values. In patients from Western countries, the progression of HCC is usually slow and is related to tumor size[2]. The mechanism for late recurrence of HCC remains unclear and some hypotheses have been proposed such as intraoperative surgical manipulation[12], embolization of tumor cells via the hepatic veins before or during liver transplantation, which can result in the trapping of micrometastasis within the capillary network of the lungs and immunosuppressive therapy potentiating macroscopic growth of nodules[5].
The effect of long-term immunosuppressive therapy on tumor growth in patients with HCC is unknown. It has been suggested that while many HCCs are growing slowly, the growth rate of recurrent tumors in patients receiving immunosuppressive therapy is significantly greater than that in those who do not receive immunosuppressive therapy, indicating that immunosuppressive therapy plays a major role in tumor recurrence after liver transplantation[9]. In fact, it has been shown that the risk of recurrence in patients who continue to receive corticosteroids may be as much as four times higher than that in patients who stop receiving corticosteroids soon after the liver transplantation[2,6].
In our case, immunosuppressive therapy seemed to be well balanced, because the patient had neither rejection nor opportunistic infections.
In conclusion, HCC metastasis can develop even after a long time of liver transplantation. A systematic long-term follow-up is necessary. In case of single lung metastasis without any other localization, it is possible to resect it, allowing to prolong the survival of the patient. It is very important to maintain vigilance after liver transplantation, because the risk of recurrence exists for a long time after liver transplantation.
Immunosuppressive therapy, one of the possible key factors in controlling the response to neoplasm, can reduce the treatment time[13] and achieve immunologic tolerance and reduce the use of immunosuppressive drugs[12].
S- Editor Wang J L- Editor Wang XL E- Editor Liu WF
| 1. | Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 850] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 2. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5299] [Article Influence: 182.7] [Reference Citation Analysis (0)] |
| 3. | Majno PE, Sarasin FP, Mentha G, Hadengue A. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: an outcome-oriented decision analysis. Hepatology. 2000;31:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 260] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 4. | Ferris JV, Baron RL, Marsh JW, Oliver JH, Carr BI, Dodd GD. Recurrent hepatocellular carcinoma after liver transplantation: spectrum of CT findings and recurrence patterns. Radiology. 1996;198:233-238. [PubMed] |
| 5. | Freise CE, Ferrell L, Liu T, Ascher NL, Roberts JP. Effect of systemic cyclosporine on tumor recurrence after liver transplantation in a model of hepatocellular carcinoma. Transplantation. 1999;67:510-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Marcellin P, Samuel D, Areias J, Loriot MA, Arulnaden JL, Gigou M, David MF, Bismuth A, Reynes M, Bréchot C. Pretransplantation interferon treatment and recurrence of hepatitis B virus infection after liver transplantation for hepatitis B-related end-stage liver disease. Hepatology. 1994;19:6-12. [PubMed] |
| 7. | Cillo U, Vitale A, Bassanello M, Boccagni P, Brolese A, Zanus G, Burra P, Fagiuoli S, Farinati F, Rugge M. Liver transplantation for the treatment of moderately or well-differentiated hepatocellular carcinoma. Ann Surg. 2004;239:150-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 257] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 8. | Mor E, Tur-Kaspa R, Sheiner P, Schwartz M. Treatment of hepatocellular carcinoma associated with cirrhosis in the era of liver transplantation. Ann Intern Med. 1998;129:643-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 173] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Yokoyama I, Carr B, Saitsu H, Iwatsuki S, Starzl TE. Accelerated growth rates of recurrent hepatocellular carcinoma after liver transplantation. Cancer. 1991;68:2095-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Marsh JW, Dvorchik I, Subotin M, Balan V, Rakela J, Popechitelev EP, Subbotin V, Casavilla A, Carr BI, Fung JJ. The prediction of risk of recurrence and time to recurrence of hepatocellular carcinoma after orthotopic liver transplantation: a pilot study. Hepatology. 1997;26:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 153] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Lam CM, Lo CM, Yuen WK, Liu CL, Fan ST. Prolonged survival in selected patients following surgical resection for pulmonary metastasis from hepatocellular carcinoma. Br J Surg. 1998;85:1198-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Suehiro T, Terashi T, Shiotani S, Soejima Y, Sugimachi K. Liver transplantation for hepatocellular carcinoma. Surgery. 2002;131:S190-S194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Vivarelli M, Bellusci R, Cucchetti A, Cavrini G, De Ruvo N, Aden AA, La Barba G, Brillanti S, Cavallari A. Low recurrence rate of hepatocellular carcinoma after liver transplantation: better patient selection or lower immunosuppression? Transplantation. 2002;74:1746-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |