Published online Aug 14, 2006. doi: 10.3748/wjg.v12.i30.4850
Revised: May 1, 2006
Accepted: May 25, 2006
Published online: August 14, 2006
AIM: To investigate whether, or how, DA-9601, which is a new gastroprotective agent, inhibits TNF-α-induced inflammatory signals in gastric epithelial AGS cells.
METHODS: Cell viability was determined by MTT assay. IL-8 and CCL20 promoter activities were determined by a luciferease reporter gene assay. NF-κB-dependent transcriptional activity was determined by I-κBα degradation, NF-κB p65 nuclear translocation and a luciferase activity assay. IL-8 and CCL20 gene expression and protein secretion were determined by RT-PCR and an enzyme-linked immunosorbent assay (ELISA). Total and phosphorylated forms of mitogen-activated protein kinases (MAPKs) were determined by Western blot.
RESULTS: Treatment of AGS cells with DA-9601 reduced TNF-α-induced IL-8 and CCL20 promoter activities, as well as their gene expression and protein release. TNF-α also induced NF-κB-dependent transcriptional activity in AGS cells. In contrast, in cells treated with DA-9601, TNF-α-induced NF-κB activity was significantly blocked. Although all three MAP kinase family members were phosphorylated in response to TNF-α, a selective inhibitor of p38 kinase SB203580 only could inhibit both NF-κB-dependent transcriptional activity and IL-8 and CCL20 production, suggesting a potential link between p38 kinase and NF-κB-dependent pathways in AGS cells. Interestingly, DA-9601 also selectively inhibited p38 kinase phosphorylation induced by TNF-α.
CONCLUSION: DA-9601 blocked TNF-α-mediated inflammatory signals by potentially modulating the p38 kinase pathway and/or a signal leading to NF-κB-dependent pathways in gastric epithelial cells.
- Citation: Choi SC, Choi EJ, Oh HM, Lee S, Lee JK, Lee MS, Shin YI, Choi SJ, Chae JR, Lee KM, Lee WJ, Park JS, Shin CY, Oh TY, Jun CD. DA-9601, a standardized extract of Artemisia asiatica, blocks TNF-α-induced IL-8 and CCL20 production by inhibiting p38 kinase and NF-κB pathways in human gastric epithelial cells. World J Gastroenterol 2006; 12(30): 4850-4858
- URL: https://www.wjgnet.com/1007-9327/full/v12/i30/4850.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i30.4850
Artemisia asiatica has been frequently used in traditional Asian medicine for the treatment of diseases such as inflammation, cancer and microbial infection. Along this line, a novel antipeptic formulation prepared from the ethanol extracts of A. asiatica, namely DA-9601 (StillenTM), has been reported to posses anti-oxidative and anti-inflammatory activities in experimentally induced gastrointestinal damage as well as hepatic and pancreatic lesions[1-4]. DA-9601 is now on the market in South Korea and will be on sale in other Asian countries in the near future. However, despite studies in animals and humans, the detailed cellular mechanism of the pharmacologic actions of DA-9601 is largely unknown.
Chemokines are potential mediators that in many cases can act as a signal for the emigration of blood cells. The C-X-C chemokines are considered the most important mediators for the accumulation of granulocytes[5,6]. One member of this cytokine family, chemokine interleukin-8 (IL-8), has been shown to be elevated in gastric biopsy samples of patients with H pylori-associated gastritis[5], and is considered to be an important mediator for the initiation of host innate immunity by recruiting granulocytes[7]. On the other hand, CCL20 is a recently described C-C chemokine (also known as a liver- and activation-regulated chemokine or macrophage inflammatory protein 3α) that was first identified by screening the GenBank database of expressed sequence tags for novel chemokine molecules[8]. CCL20 is also expressed in gastric epithelial cells, upregulated by infection with H pylori, and implicated in the initiation of host adaptive immunity by regulating recruitment of dendritic cells[9], effector memory T cells and B cells via CCR6[10]. Given their potential importance in inflammatory responses, these two chemokines may be good target systems in evaluating the anti-inflammatic efficacy of potential pharmacologic drugs in gastric epithelial cells.
In the current study, we primarily investigated whether TNF-α induces IL-8 and CCL20 genes, as well as their protein products, in human gastric epithelial AGS cells. Although TNF-α is a candidate factor for involvement in inflammation-mediated gastric mucosal injury, the mechanisms of action for this cytokine on gastric epithelial cells are still poorly understood. We next analyzed where DA-9601 acted in the TNF-α-induced inflammatory cascade.
DA-9601 (Lot No. DA-9601-L-07) with 0.42% of active ingredient, eupatilin[11], was extracted from A. asiatica and supplied to this study after HPLC analysis in Dong-A Pharmaceutical Co. Ltd., (Yongin, South Korea)[2]. Alkaline phosphatase-conjugated rabbit anti-goat IgG, and p-nitrophenyl phosphate tablets, dimethyl sulfoxide, phosphate-buffered saline (PBS), 3-(4, 5,-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) were purchased from Sigma Chemical Co. (St. Louis, MO). Recombinant human TNF-α goat anti-human IL-8 polyclonal antibody, mouse anti-human CCL20 monoclonal antibody (clone 67310.111), and goat anti-human CCL20 polyclonal antibody were obtained from R&D Systems Inc. (Minneapolis, MN). Rabbit anti-human IL-8 polyclonal antibody was from Endogen Inc. (Woburn, MA). Antibodies against p38 kinase, c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), and the antibodies specific to the phosphorylated forms (pp38, Thr180/Tyr182; pJNK, Thr183 Tyr185; pERK1/2, Thr202/Tyr204) were purchased from Cell Signaling Technology, Inc.(Beverly, MA). SB203580, SP600125, PD98059 and PDTC were purchased from Calbiochem (La Jolla, CA). Anti-human I-κBα was from Santa Cruz Biotechnology (Santa Cruz, CA).
IL-8 promoter-luciferase reporter vector (pGL3-pIL-8) was obtained from Dr. J.-S. Chun in Gwangju Institute of Science and Technology (Korea). The CCL20 promoter from -1905 to +30 was amplified from 100 ng of human genomic DNA by PCR under standard conditions with the following primers (restriction sites underlined) pCCL20_forward(SacI) 5’-ATACCGAGCTCGGCCAGTCTGGTCTCGAACT-3’; pCCL20_reverse (HindIII) 5’- ATACCAAGCTTCTTTAATCAATATTGCAGTT-3’ and cloned into the pGL3-basic plasmid (Promega, Mannheim, Germany) to generate pGL3-pCCL20 luciferase vector. pGL3-pCCL20 was sequence verified with an ABI3700 sequencer (ABI, Foster City, CA) before use.
Human gastric epithelial AGS cells and human kidney epithelial 293T were obtained from the American Type Culture Collection (ATCC). The cells were cultured at 37°C in a 5% CO2 atmosphere in RPMI-1640 supplemented with heat-inactivated 10% fetal bovine serum (FBS; GibcoBRL) and appropriate antibiotics.
Cellular viability was evaluated by the reduction of MTT to formazan. A stock solution of MTT was prepared in phosphate-buffered saline (PBS), diluted in RPMI 1640 medium, and added to cell-containing wells at a concentration of 0.5 mg/mL after the culture medium was first removed. The plates were then incubated for 4 h at 37°C in 5% CO2. At the end of the incubation period, the medium was aspirated, and the formazan product was solubilized with dimethyl sulfoxide. Absorbency was measured on a multiscan reader with a 570 nm wavelength filter.
The concentration of IL-8 or CCL20 in culture supernatants from AGS cells was measured by a previously described method[12,13]. In brief, 96-well microtiter plates (MaxiSorpTM, Nunc, Denmark) were coated with 2 μg/mL of goat anti-human IL-8 (R&D Systems) or mouse anti-human CCL20 (clone 67310.111; R&D Systems) in 50 μL PBS at 4°C overnight. All further steps were carried out at room temperature. After washing three times with PBS, non-specific binding sites were blocked by incubation with 150 μL PBS, 1% BSA and 0.05% Tween 20/well for 2 h. After three washes with PBS, 50 μL of samples or standards were added and incubated for 2 h. As a second antibody, 0.5 μg/mL polyclonal rabbit anti-human IL-8 (Endogen) or polyclonal goat anti-human CCL20 (R&D Systems) was added and incubated for 2 h. As a third antibody, alkaline phosphatase-conjugated monoclonal mouse anti-rabbit IgG (for IL-8) or rabbit anti-goat IgG (for CCL20) was diluted in 50 μL PBS 0.1% BSA and 0.05% Tween 20 to 1:50 000 and incubated for 2 h. Finally, alkaline phosphatase substrate p-nitrophenyl phosphate (Sigma) was added at a concentration of 1mg/mL in 0.1 mol/L glycine buffer, pH 10.4, containing 1 mol/L MgCl2 and 1 mol/L ZnCl2. After overnight incubation, plates were read at 405 nm on a microplate reader (Molecular Devices Corp., Sunnyvale, CA). The detection limit of the ELISA was 30 pg/mL.
AGS cells (5 × 106) were grown in 60-mm culture dish and were incubated for 16 h in a fresh medium containing stimuli as indicated. After discarding the growth medium, total RNA was isolated from cells using easy Blue (iNtRON Biotechnology, Daejon, Korea), following the manufacturer’s instructions. Reverse transcription of the RNA was performed using AccuPower RT PreMix (Bioneer, Daejon, Korea). One microgram of RNA and 20 pmol primers were preincubated at 70°C for 5 min and transferred to a mixture tube. The reaction volume was 20 μL. cDNA synthesis was performed at 42°C for 60 min, followed by RT inactivation at 94°C for 5 min. Thereafter, the RT-generated DNA (2-5 μL) was amplified using AccuPower® RT PreMix (Bioneer, Korea). The primers used for cDNA amplification were: 5’-ATGACTTCCAAGCTGGCCGTGGCT-3’ (sense) and 5’-TCTCAGCCCTCTTCAAAAACTTCTC-3’ (antisense) for IL-8[14]; 5’-ATGTGCTGTACCAAGAGTTTG-3’ (sense) and 5’-TTACATGTTCTTGACTTTTTTACTGAGGAG-3’ (antisense) for CCL20[15]; 5’-CGGAGTCAACGGATTTGGTCGTAT-3’ (sense), 5’-AGCTTCTCCATGGTGGTGAAGAC-3’ (antisense) for GAPDH[12]. Amplification conditions were denaturation at 94°C for 30 s, annealing at 56°C for 30 s, and extension at 72°C for 30 s for 30 cycles. The expected PCR products were 289 bp (for IL-8), 291 bp (for CCL20), and 306 bp (for GAPDH). PCR products were subjected to electrophoresis on 1.2 % agarose gel and were stained with ethidium bromide.
AGS cells were seeded at 5 × 105 in a 4-well plate 1 d before transfection (50% cell confluency). Cells were transfected with serum- and antibiotics-free RPMI 1640 medium containing 4 μg/mL Lipofectamine 2000 reagent (Invitrogen) and 1.6 μg/mL of NF-κB p65-EGFP vector (provided by Prof. Rainder de Martin, Department Vascular Pharmacology and Thrombosis Research, University of Vienna, Austria). After 5 h of incubation, medium was replaced with RPMI 1640 medium containing 10% FBS and antibiotics. Cells were allowed to recover at 37°C for 20 h and subsequently were stimulated as indicated in the text or figures. Fluorescence images were observed under the Olympus microscopy (Melville, NY).
For the analysis of phosphorylated or total protein levels of mitogen-activated protein kinases (MAPKs) and the I-κB degradation, stimulated cells were rinsed twice with ice-cold phosphate-buffered saline and then lysed in ice-cold lysis buffer (50 mmol/L Tris-HCl, pH 7.4, containing 150 mmol/L NaCl, 1% Nonidet P-40, 0.1% SDS, 0.1% deoxycholate, 5 mmol/L sodium fluoride, 1 mmol/L sodium orthovanadate, 1 mmol/L 4 nitrophe-nylphosphate, 10 g/mL of leupeptin, 10 μg/mL of pepstatin A, and 1 mmol/L 4-(2 aminoethyl) Benzenee Sufonyl fluoride). Cell lysates were centrifuged at 15 000 rpm for 20 min at 4°C, and the supernatant was mixed with a one-fourth volume of 4 × SDS sample buffer, boiled for 5 min, and then separated through a 12% SDS-PAGE gel. After electrophoresis, proteins were transferred to a nylon membrane by means of Trans-Blot SD semi-dry transfer cell (Bio-Rad, Hercules, CA). The membrane was blocked in 5% skim milk (1 h), rinsed, and incubated with primary antibody (for phosphorylated MAP kinases or I-κB) in TBS containing 0.05% Tween 20 (TBS-T) and 3% skim milk overnight at 4°C. Excess primary antibody was then removed by washing the membrane four times in TBS-T, and the membrane was incubated with 0.1 μg/mL peroxidase-labeled secondary antibody (against rabbit) for 1 h. Following three washes in TBS-T, bands were visualized by ECLTM Western Blotting Detection reagents and exposed to X-ray film.
For transient transfections, AGS cell and 293T Cells were seeded at 5 × 105 in a 12-well plate 1 d before transfection (90%-95% cell confluency). Cells were transfected with serum- and antibiotics-free RPMI 1640 medium containing 4 μg/mL Lipofectamine 2000 reagent (Invitrogen) and 1.6 μg/mL of NF-κB, IL-8 (provided by Professor J.-S. Chun, Gwangju Institute of Science and Technology, Gwangju, Korea), or CCL20 luciferase reporter constructs. After 5 h of incubation, medium was replaced with RPMI 1640 medium containing 10% FBS and antibiotics. Cells were allowed to recover at 37°C for 20 h and subsequently were stimulated as indicated in the text or figures. Cell lysates were prepared and assayed for luciferase activity using Luciferase Assay System (Promega, Madison, WI), according to the manufacturer’s instructions.
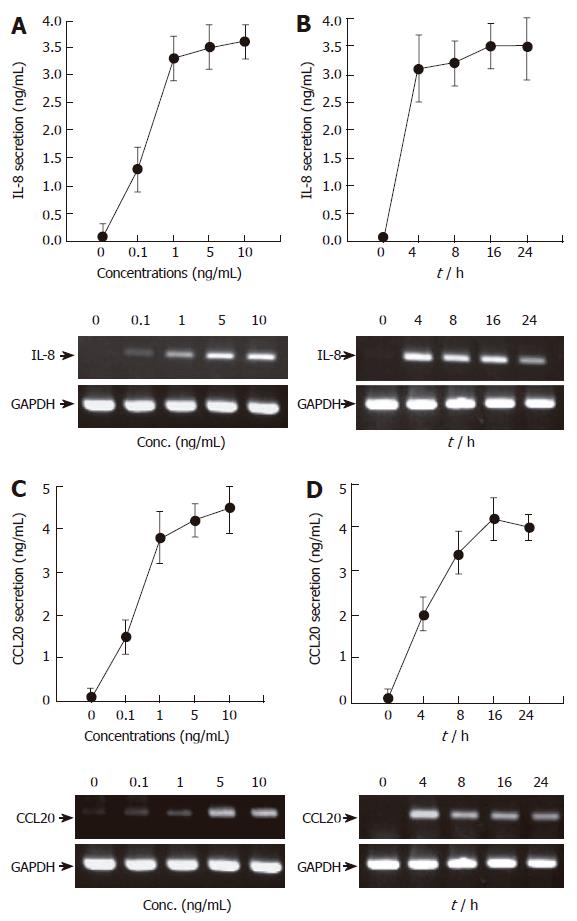
One of the key molecules mediating the gastric mucosal inflammation is the cytokine TNF-α[16]. We therefore first examined whether TNF-α induces inflammatory signals in AGS cells. We were primarily interested in two chemokines; i.e., IL-8 and CCL20, because these proteins play central roles in the evocation of host innate and adaptive immunity[10,17]. Treatment of AGS cells with TNF-α markedly induced IL-8 and CCL20 secretion (Figure 1). The effect of TNF-α was concentration-dependent in the range of 0-10 ng/mL, as assessed by ELISA and RT-PCR. We chose 5 ng/mL of TNF-α for the following experiments as this concentration is enough for maximal induction of IL-8 and CCL20 (Figure 1A and C). Time-dependent experiments revealed that treatment with TNF-α led to rapid induction of IL-8 and CCL20 mRNAs (about 4 h after stimulation) while their protein secretions were slightly delayed (about 16 h after stimulation) in AGS cells. Overall, we conclude that AGS cells produce IL-8 and CCL20 in response to TNF-α.
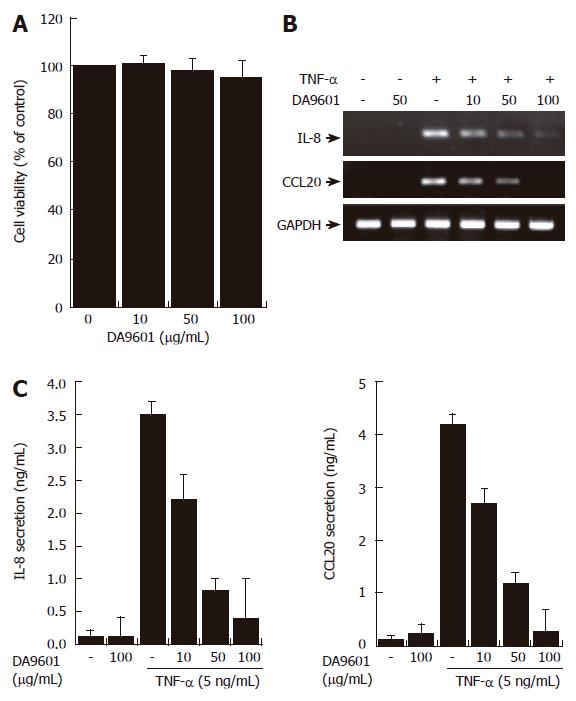
According to previous reports the main effect of DA-9601 is associated with cell death or apoptosis in the rat model of cerulein-induced pancreatitis[1]. To test preliminarily whether DA-9601 affects viability of human gastric epithelial AGS cells, we performed a MTT assay. The concentrations that ranged from 0 to 100 μg/mL of DA-9601 showed no toxic effects on AGS cells at 16 h of incubation, while higher concentrations of DA-9601 (>100 μg/mL) induced delayed cytotoxicity after 24 h (Figure 2A and data not shown). There were no detectable apoptotic nuclei in DA-9601 (0-100 μg/mL)-treated cells, as verified by DAPI staining (data not shown).
RT-PCR revealed that DA-9601 (0-100 μg/mL), which alone did not induce any significant changes, significantly attenuated TNF-α (5 ng/mL)-dependent expression of IL-8 and CCL20 mRNA in human AGS cells (Figure 2B). Addition of DA-9601 dramatically reduced TNF-α-induced IL-8 and CCL20 secretions as well in a dose-dependent manner (Figure 1C). The concentration of 100 μg/mL of DA-9601 maximally inhibited the secretion of both chemokines; i.e., IL-8 and CCL20 (Figure 1C). However, as this concentration revealed weak cytotoxicity after 24 h of treatment (data not shown), we therefore chose 50 μg/mL of DA-9601 for the following experiments, unless otherwise indicated.
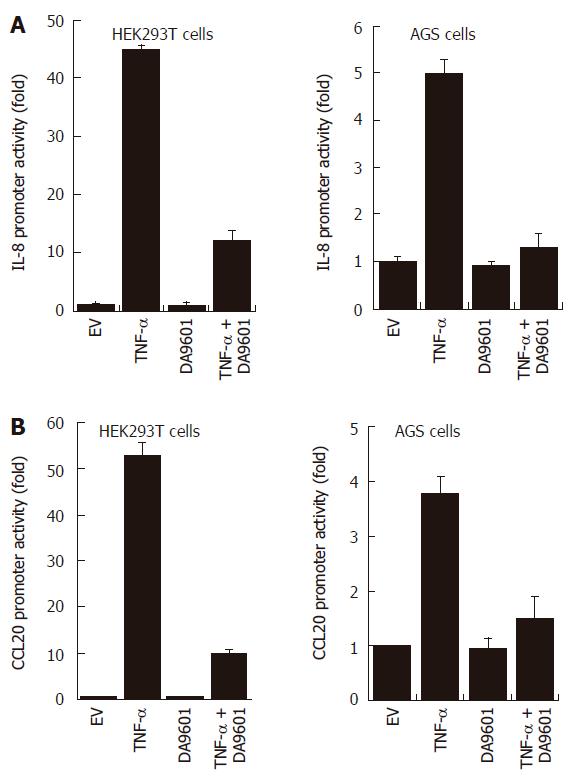
To investigate whether the inhibition of both chemokine secretions by DA-9601 is due to the direct down-regulation of promoter activity, we performed the luciferase reporter gene assay for IL-8 and CCL20 promoters. As shown in Figure 3, treatment with TNF-α significantly induced IL-8 and CCL20 promoter activities (promoter-dependent luciferase signals) in both HEK293T cells and AGS cells. However, pre-incubation of these cells with DA-9601 (50 μg/mL) dramatically reduced TNF-α-induced promoter activities in both cell types, suggesting that DA-9601 inhibits IL-8 and CCL20 expressions and secretions via direct or indirect modulation of promoter activities.
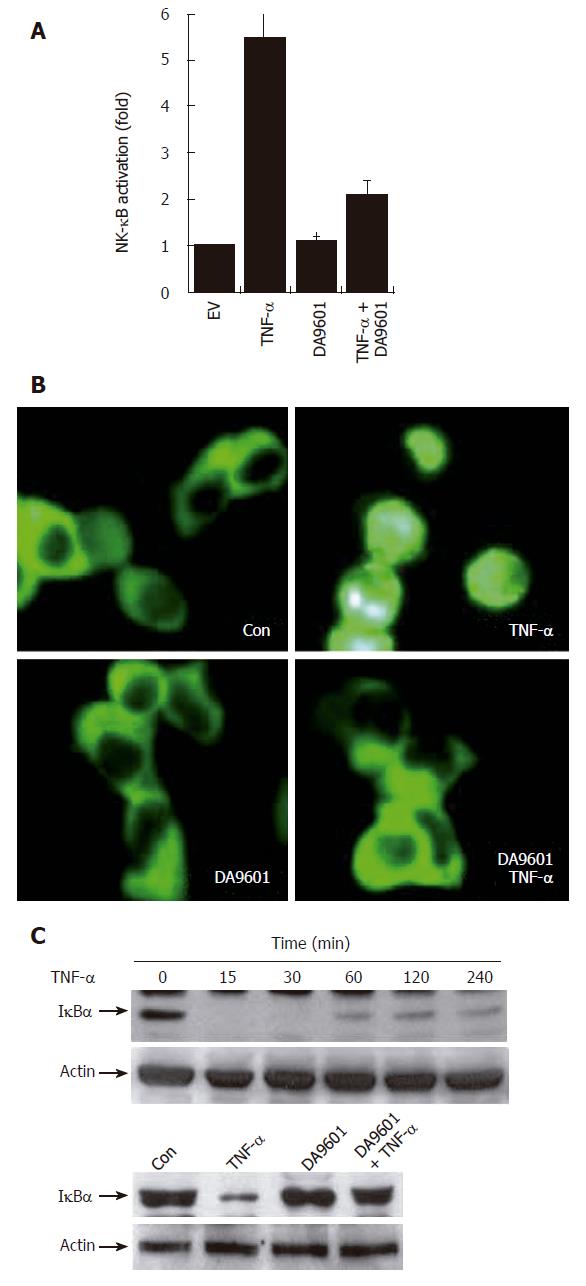
Several recent studies have demonstrated that gene expression of both IL-8 and CCL20 is NF-κB dependent[18-21]. This led us to examine whether DA-9601 can inhibit NF-κB activity in TNF-α-treated AGS cells. We therefore examined the NF-κB activation by measuring NF-κB-dependent transcriptional activity, NF-κB p65 nuclear translocation, and I-κBα degradation. As shown in Figure 4A and B, incubation of AGS cells with DA-9601 for 24 h significantly decreased TNF-α-induced luciferase activity. We next measured nuclear translocation of the NF-κB p65 subunit. To this end, AGS cells were transfected with NF-κB-p65-EGFP vector for 24 h, and then the cells were further treated with DA-9601 (50 μg/mL), TNF-α (5 ng/mL), or DA-9601 (1 h before TNF-α treatment) plus TNF-α. As shown in Figure 3C, DA-9601 significantly inhibited nuclear translocation of NF-κB p65 subunit after 1 h of TNF-α treatment. We finally tested whether DA-9601 inhibits I-κBα degradation in TNF-α-treated AGS cells. Treatment with TNF-α rapidly induced I-κB degradation (about 15 min) which later recovered slightly (> 240 min) (Figure 4C). However, pre-incubation of AGS cells with DA-9601 (1 h) significantly inhibited TNF-α-induced I-κBα degradation (Figure 4B). Taken together, we conclude that DA-9601 inhibits IL-8 and CCL20 expressions and their protein releases, presumably by acting at the site or upstream of NF-κB-dependent pathways.
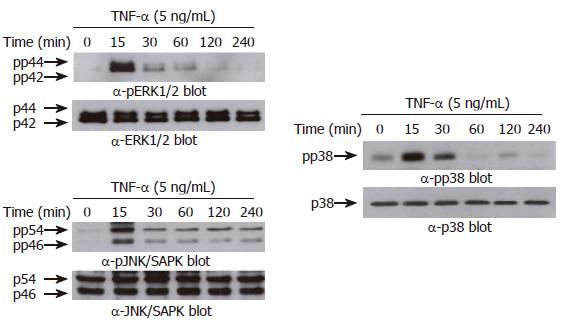
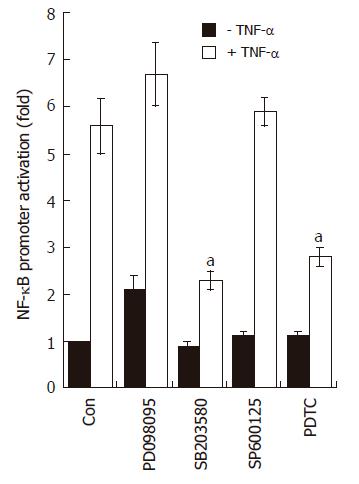
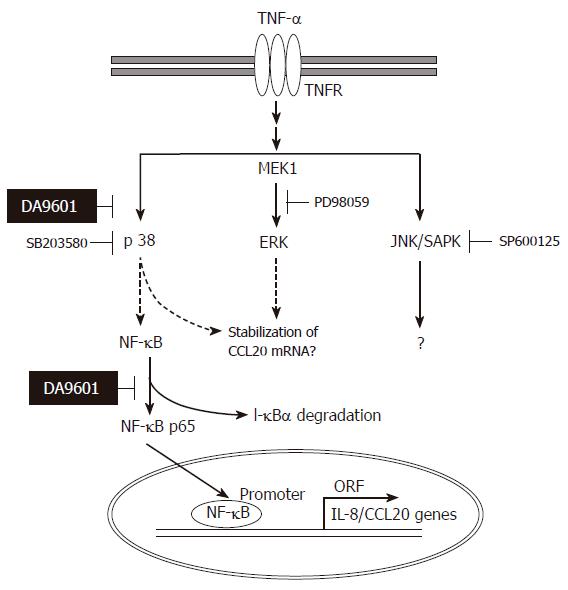
Previous reports demonstrated that three structurally-related mitogen-activated protein kinases (MAPKs) play crucial roles in a variety of systems involving TNF-α[22-24]. We therefore asked whether TNF-α leads to phosphorylation of the MAPK subfamilies in AGS cells. As shown in Figure 5, treatment with TNF-α (5 ng/mL) rapidly induced phosphorylation of all three MAPK subfamilies. The maximal phosphorylation levels were achieved as early as 15 min in all MAPKs after TNF-α treatment, and thereafter the levels were gradually decreased. Interestingly, however, among three MAPK blockers used, the selective p38 kinase inhibitor SB203580 (10 μmol/L) could only inhibit TNF-α-induced NF-κB-dependent promoter activity (Figure 6). As expected, treatment with PDTC (10 μmol/L) also inhibited NF-κB-dependent promoter activity (Figure 6). These results suggest a functional cross-talk between p38 kinase and NF-κB-dependent signaling system, and further suggest that p38 kinase acts upstream of NF-κB activation, thereby inhibiting IL-8 and CCL20 promoter activities in AGS cells.
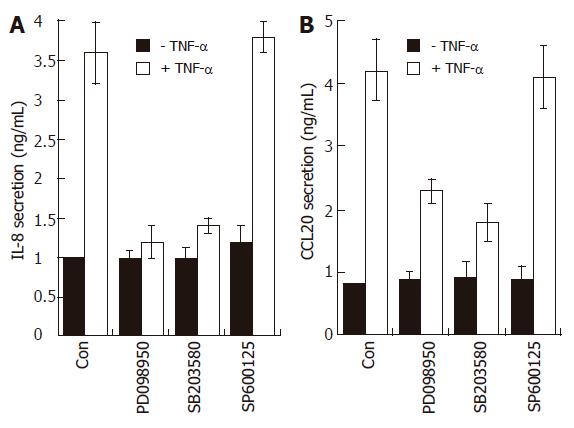
To further confirm that p38 kinase is involved in chemokine production, AGS cells were incubated with three MAPK inhibitors prior to TNF-α treatment and then the production of IL-8 and CCL20 was measured by the ELISA method. As expected, treatment with SB203580 significantly inhibited IL-8 and CCL20 production induced by TNF-α (Figure 7). The selective inhibitor of the MEK1 pathway PD098059 also inhibited IL-8 and CCL20 production in TNF-α-treated AGS cells, while it had no effect on NF-κB-dependent transcriptional activity (Figure 6). These results may suggest that, in terms of IL-8 or CCL20 production, ERK1/2 is not coupled with NF-κB-dependent pathways but may act at a post-transcriptional level.
An important question raised at this point was whether DA-9601 also inhibits p38 kinase phosphorylation induced by TNF-α. To test this, AGS cells were treated with DA-9601 for 1 h, and then the cells were further incubated for 15 min with TNF-α (5 ng/mL). The phosphorylation levels of all three MAPKs were determined by Western blot analysis. Surprisingly, while having no effect on both ERK1/2 and JNK1/2, DA-9601 specifically and significantly inhibited p38 kinase phosphorylation induced by TNF-α (Figure 8). These results strongly suggest that DA-9601 inhibits TNF-α-induced chemokine production as well as secretion via a direct or indirect inhibition of p38 kinase activity. Further, these results suggest that DA-9601 inhibits NF-κB-dependent transcriptional activity presumably by blocking the p38 kinase signaling system.
In this study we analyzed the effect and mechanism of action whereby DA-9601 modulates the production of two chemokines; i.e., IL-8 and CCL20, in human gastric epithelial AGS cells. IL-8 is one of the key molecules that is involved in innate immunity, and is known to be elevated in gastric biopsy samples of patients with H pylori-associated gastritis[5]. In contrast, it was generally accepted that gastric epithelial cells do not produce the cytokines that are essential components of host adaptive immunity. However, recent study has shown that they do produce CCL20 upon infection with H pylori[9], thereby suggesting that CCL20 is also involved in gastric mucosal immunity. The present results demonstrate that AGS cells do produce both IL-8 and CCL20 chemokines in response to TNF-α stimulation. This evidence extends the current view and suggests that gastric epithelial cells may also have a critical function by inducing mucosal innate immunity as well as adaptive immunity.
The use of natural anti-inflammatory products provides an attractive and safe alternative to modulate inflammatory disorders. A. asiatica has been widely used for centuries in traditional Asian diets and medications without any serious side effects. Also, the standardized ethanol extract (DA-9601) of this medicinal plant has been shown to have strong antioxidative and anti-inflammatory effects in experimental animal models, such as esophageal mucosal damage[25,26], ethanol-induced gastritis[4], and cerulin-induced pancreatitis[1]. However, the mechanisms of action of DA-9601 in vitro and in vivo are still unclear. Our data indicate that TNF-α-mediated expression of chemokine genes in gastric epithelial cells is blocked by DA-9601 treatment. The mechanism of action of DA-9601 involves blockade of NF-κB activation, in agreement with previous studies using a mouse skin model[27]. To further define the mechanism by which NF-κB activity is inhibited by DA-9601, we investigated molecular relationships between MAPKs and NF-κB. We found that SB203580, a selective inhibitor of p38 kinase, blocked NF-κB activity, thereby suggesting that p38 kinase may be functionally linked with NF-κB. In this regard, it is particularly interesting to note that DA-9601 could block activation of both p38 kinase and NF-κB in AGS cells. This suggests that DA-9601 does not directly block the NF-κB-dependent signaling system, but instead, it may indirectly inhibit NF-κB through the inhibition of p38 kinase pathways. Accordingly, several lines of evidence have suggested that p38 kinase lies upstream of NF-κB[28-30]. It is also interesting that PD098059, the upstream inhibitor of ERK1/2 pathway, had no effect on TNF-α-induced NF-κB activity, while it significantly blocked IL-8 and CCL20 production. These results suggests that the ERK1/2 pathway is not involved in the regulation of promoter activity but may participate in the stabilization of chemokine genes, as demonstrated by other reports[12,31].
Although DA-9601 has substantial anti-inflammatory or anti-oxidative effects, the structural identity of its active component(s) remains to be elucidated. Eupatilin, one of the major pharmacologically active ingredients of A. asiatica, may share its anti-inflammatory[32] or anti-oxidative effects[33] with DA-9601. However, our unpublished results demonstrated that eupatilin has no significant effect on TNF-α-induced IL-8 expression and secretion, while it has strong protective (anti-oxidative) effect for AGS cells from hydrogen peroxide-induced cellular damage (data not shown). This implies that DA-9601 may also have other active ingredient(s) that selectively inhibit(s) cytokine-induced expression or release of IL-8 and CCL20 proteins as well as other inflammation-related proteins.
Collectively, the data obtained in the present study are compatible with the schematic representation in Figure 9. DA-9601 has an anti-inflammatory potential based on its blocking effects on TNF-α-induced IL-8 and CCL20 production. The inhibition by DA-9601 appears to be mediated through the inhibition of promoter activity as well as the NF-κB-dependent signaling system. Inhibition of p38 kinase by SB203580 blocked NF-κB activity, suggesting that p38 kinase is functionally linked with NF-κB and lies upstream of NF-κB. More interestingly, DA-9601 inhibited activation of both the p38 kinase and NF-κB-dependent systems. This suggests that DA-9601 inhibits NF-κB directly or indirectly through the inhibition of the p38 kinase pathway. Additional studies will be required to clarify the upstream signal transduction pathways that might be affected by DA-9601.
Co-first-author: Eun-Ju Choi
S- Editor Wang J L- Editor Lutze M E- Editor Ma WH
| 1. | Hahm KB, Kim JH, You BM, Kim YS, Cho SW, Yim H, Ahn BO, Kim WB. Induction of apoptosis with an extract of Artemisia asiatica attenuates the severity of cerulein-induced pancreatitis in rats. Pancreas. 1998;17:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Ryu BK, Ahn BO, Oh TY, Kim SH, Kim WB, Lee EB. Studies on protective effect of DA-9601, Artemisia asiatica extract, on acetaminophen- and CCl4-induced liver damage in rats. Arch Pharm Res. 1998;21:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Oh TY, Lee JS, Ahn BO, Cho H, Kim WB, Kim YB, Surh YJ, Cho SW, Lee KM, Hahm KB. Oxidative stress is more important than acid in the pathogenesis of reflux oesophagitis in rats. Gut. 2001;49:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Huh K, Kwon TH, Shin US, Kim WB, Ahn BO, Oh TY, Kim JA. Inhibitory effects of DA-9601 on ethanol-induced gastrohemorrhagic lesions and gastric xanthine oxidase activity in rats. J Ethnopharmacol. 2003;88:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Cole SP, Cirillo D, Kagnoff MF, Guiney DG, Eckmann L. Coccoid and spiral Helicobacter pylori differ in their abilities to adhere to gastric epithelial cells and induce interleukin-8 secretion. Infect Immun. 1997;65:843-846. [PubMed] |
| 6. | Rieder G, Hatz RA, Moran AP, Walz A, Stolte M, Enders G. Role of adherence in interleukin-8 induction in Helicobacter pylori-associated gastritis. Infect Immun. 1997;65:3622-3630. [PubMed] |
| 7. | Agace W, Hedges S, Andersson U, Andersson J, Ceska M, Svanborg C. Selective cytokine production by epithelial cells following exposure to Escherichia coli. Infect Immun. 1993;61:602-609. [PubMed] |
| 8. | Hieshima K, Imai T, Opdenakker G, Van Damme J, Kusuda J, Tei H, Sakaki Y, Takatsuki K, Miura R, Yoshie O. Molecular cloning of a novel human CC chemokine liver and activation-regulated chemokine (LARC) expressed in liver. Chemotactic activity for lymphocytes and gene localization on chromosome 2. J Biol Chem. 1997;272:5846-5853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 290] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 9. | Nishi T, Okazaki K, Kawasaki K, Fukui T, Tamaki H, Matsuura M, Asada M, Watanabe T, Uchida K, Watanabe N. Involvement of myeloid dendritic cells in the development of gastric secondary lymphoid follicles in Helicobacter pylori-infected neonatally thymectomized BALB/c mice. Infect Immun. 2003;71:2153-2162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 618] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 11. | Seo HJ, Surh YJ. Eupatilin, a pharmacologically active flavone derived from Artemisia plants, induces apoptosis in human promyelocytic leukemia cells. Mutat Res. 2001;496:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Choi EY, Kim EC, Oh HM, Kim S, Lee HJ, Cho EY, Yoon KH, Kim EA, Han WC, Choi SC. Iron chelator triggers inflammatory signals in human intestinal epithelial cells: involvement of p38 and extracellular signal-regulated kinase signaling pathways. J Immunol. 2004;172:7069-7077. [PubMed] |
| 13. | Sierro F, Dubois B, Coste A, Kaiserlian D, Kraehenbuhl JP, Sirard JC. Flagellin stimulation of intestinal epithelial cells triggers CCL20-mediated migration of dendritic cells. Proc Natl Acad Sci USA. 2001;98:13722-13727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 275] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 14. | Németh ZH, Deitch EA, Szabó C, Fekete Z, Hauser CJ, Haskó G. Lithium induces NF-kappa B activation and interleukin-8 production in human intestinal epithelial cells. J Biol Chem. 2002;277:7713-7719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Scapini P, Laudanna C, Pinardi C, Allavena P, Mantovani A, Sozzani S, Cassatella MA. Neutrophils produce biologically active macrophage inflammatory protein-3alpha (MIP-3alpha)/CCL20 and MIP-3beta/CCL19. Eur J Immunol. 2001;31:1981-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Bodger K, Crabtree JE. Helicobacter pylori and gastric inflammation. Br Med Bull. 1998;54:139-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 134] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Yoshikawa T, Naito Y. The role of neutrophils and inflammation in gastric mucosal injury. Free Radic Res. 2000;33:785-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Jobin C, Sartor RB. The I kappa B/NF-kappa B system: a key determinant of mucosalinflammation and protection. Am J Physiol Cell Physiol. 2000;278:C451-C462. [PubMed] |
| 19. | Izadpanah A, Dwinell MB, Eckmann L, Varki NM, Kagnoff MF. Regulated MIP-3alpha/CCL20 production by human intestinal epithelium: mechanism for modulating mucosal immunity. Am J Physiol Gastrointest Liver Physiol. 2001;280:G710-G719. [PubMed] |
| 20. | Harant H, Eldershaw SA, Lindley IJ. Human macrophage inflammatory protein-3alpha/CCL20/LARC/Exodus/SCYA20 is transcriptionally upregulated by tumor necrosis factor-alpha via a non-standard NF-kappaB site. FEBS Lett. 2001;509:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Imaizumi Y, Sugita S, Yamamoto K, Imanishi D, Kohno T, Tomonaga M, Matsuyama T. Human T cell leukemia virus type-I Tax activates human macrophage inflammatory protein-3 alpha/CCL20 gene transcription via the NF-kappa B pathway. Int Immunol. 2002;14:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Sluss HK, Barrett T, Dérijard B, Davis RJ. Signal transduction by tumor necrosis factor mediated by JNK protein kinases. Mol Cell Biol. 1994;14:8376-8384. [PubMed] |
| 23. | Minden A, Lin A, McMahon M, Lange-Carter C, Dérijard B, Davis RJ, Johnson GL, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 882] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 24. | Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420-7426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1750] [Cited by in RCA: 1812] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 25. | Lee JS, Oh TY, Ahn BO, Cho H, Kim WB, Kim YB, Surh YJ, Kim HJ, Hahm KB. Involvement of oxidative stress in experimentally induced reflux esophagitis and Barrett's esophagus: clue for the chemoprevention of esophageal carcinoma by antioxidants. Mutat Res. 2001;480-481:189-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Oh TY, Lee JS, Ahn BO, Cho H, Kim WB, Kim YB, Surh YJ, Cho SW, Hahm KB. Oxidative damages are critical in pathogenesis of reflux esophagitis: implication of antioxidants in its treatment. Free Radic Biol Med. 2001;30:905-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Seo HJ, Park KK, Han SS, Chung WY, Son MW, Kim WB, Surh YJ. Inhibitory effects of the standardized extract (DA-9601) of Artemisia asiatica Nakai on phorbol ester-induced ornithine decarboxylase activity, papilloma formation, cyclooxygenase-2 expression, inducible nitric oxide synthase expression and nuclear transcription factor kappa B activation in mouse skin. Int J Cancer. 2002;100:456-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Hippenstiel S, Soeth S, Kellas B, Fuhrmann O, Seybold J, Krüll M, Eichel-Streiber C, Goebeler M, Ludwig S, Suttorp N. Rho proteins and the p38-MAPK pathway are important mediators for LPS-induced interleukin-8 expression in human endothelial cells. Blood. 2000;95:3044-3051. [PubMed] |
| 29. | Read MA, Whitley MZ, Gupta S, Pierce JW, Best J, Davis RJ, Collins T. Tumor necrosis factor alpha-induced E-selectin expression is activated by the nuclear factor-kappaB and c-JUN N-terminal kinase/p38 mitogen-activated protein kinase pathways. J Biol Chem. 1997;272:2753-2761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 286] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 30. | Nick JA, Avdi NJ, Young SK, Lehman LA, McDonald PP, Frasch SC, Billstrom MA, Henson PM, Johnson GL, Worthen GS. Selective activation and functional significance of p38alpha mitogen-activated protein kinase in lipopolysaccharide-stimulated neutrophils. J Clin Invest. 1999;103:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 236] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 31. | Jijon HB, Panenka WJ, Madsen KL, Parsons HG. MAP kinases contribute to IL-8 secretion by intestinal epithelial cells via a posttranscriptional mechanism. Am J Physiol Cell Physiol. 2002;283:C31-C41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Koshihara Y, Neichi T, Murota S, Lao A, Fujimoto Y, Tatsuno T. Selective inhibition of 5-lipoxygenase by natural compounds isolated from Chinese plants, Artemisia rubripes Nakai. FEBS Lett. 1983;158:41-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Heo HJ, Cho HY, Hong B, Kim HK, Kim EK, Kim BG, Shin DH. Protective effect of 4',5-dihydroxy-3',6,7-trimethoxyflavone from Artemisia asiatica against Abeta-induced oxidative stress in PC12 cells. Amyloid. 2001;8:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |