Published online Jan 21, 2006. doi: 10.3748/wjg.v12.i3.479
Revised: June 8, 2005
Accepted: June 15, 2005
Published online: January 21, 2006
AIM: To investigate the intestinal barrier changes in rats with CCl4-induced portal hypertension.
METHODS: The permeability of intestinal barrier detected by Lanthanum as a tracer was evaluated in rats. Bacterial translocation and plasma endotoxin were also determined.
RESULTS: The incidence of bacterial translocation was 85% in rats with CCl4-induced portal hypertension, which was significantly higher than that in control rats (20%, P<0.01). Plasma endotoxin level was significantly higher in experimental group than in control group. Permeability of the epithelial mucosa and pathological alteration were increased in the ileum and the microvilli became shorter and thinner in rats with portal hypertension.
CONCLUSION: Bacterial translocation occurs in rats with CCl4-induced portal hypertension and increased permeability between epithelial cells contributes to the translocation.
- Citation: Yao GX, Shen ZY, Xue XB, Yang Z. Intestinal permeability in rats with CCl4-induced portal hypertension. World J Gastroenterol 2006; 12(3): 479-481
- URL: https://www.wjgnet.com/1007-9327/full/v12/i3/479.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i3.479
The incidence of bacterial infection in patients with portal hypertension is higher than that in the general population[1]. Most of these infections are caused by bacteria normally present in the intestine. Portal hypertension may play an important role in the pathogenesis of infections in cirrhotic patients[2]. On the other hand, endotoxemia present in patients with portal hypertension is correlated with the severity of the disease. Recently, bacterial translocation from the gut to extraintestinal organs and systemic circulation has been proved in patients with other diseases[3] and is considered as the cause of endotoxemia in patients due to the disruption of the gut barrier function. However, the exact route of bacterial translocation and endotoxemia in patients with portal hypertension is not clear. In the present study, bacterial translocation, permeability of the enterocyte membrane and intestinal morphological alterations in rats with CCl4-induced portal hypertension were evaluated by transmission electroscopy.
Adult male Sprague-Dawley rats (n = 40) weighing 250-300 g were used. The animals were allowed to acclimate to laboratory condition for 4-6 d with free access to water and rat chow before the experiment.
Twenty rats entered the experimental group and received intragastric CCl4 via a Hamilton syringe with an attached stainless steel animal feeding tube after light ether anesthesia. The first dose of CCl4 was 20 μL and subsequent doses were adjusted based on the body weight 48 h after the last dose. After the appearance of ascites, the dose was reduced to 40 μL and increased according to the schedule if ascites resolved. The other 20 rats were gavaged with water and served as control group.
Studies on intestinal permeability and bacterial translocation from the gut were carried out on d 14. The abdominal cavity was opened by a midline incision and blood samples were immediately inoculated into 3 mL blood culture medium and 0.5 mL plasma was used to detect endotoxin. Limulus-amoebocyte-lysate (LAL) test was used to determine plasma endotoxin levels as previously described[4]. Escherichia coli 055 was used as endotoxin standard. The results were expressed as endotoxin units per milliliter (EU/mL). The mesenteric lymph nodes (MLNs) near the distal ileum, liver, and spleen were harvested and weighed, tissues were then homogenized in a test tube containing 3 mL brain heart infusion broth, 0.2 mL supernatant was taken for bacterial culture. All media for aerobic culture were incubated at 37 °C for 3-5 d and isolated bacteria were identified by standard procedures. At the end of the experiment, a segment of the distal ileum was removed and placed into 2.5% glutaraldehyde fixative with 4% lanthanum hydroxide for 1 h, then washed thrice to assay the permeability of the intestinal barrier. Sections were made from each sample and the presence of the tracer was observed under transmission electron microscope.
Positive MLN culture was considered indicative of bacterial translocation from the gut. Positive blood, spleen or liver cultures were considered indicative of passage bacteria to the systemic circulation.
Translocation incidence was analyzed by the Fisher’s exact test. Weights and other data were expressed as mean ± SD and compared with Student’s t test.
The mean spleen weight in rats with CCl4-induced portal hypertension (5.32 ± 0.38 mg/g body wt) was significantly greater than that in control rats (2.31 ± 0.28 mg/g body wt). The weight of MLN was also greater in rats with CCl4-induced portal hypertension (1.85 ± 0.42 mg/g body wt) than that in control rats (0.86 ± 0.25 mg/g body wt). Ascites occurred in all rats.
Bacterial translocation to MLN occurred in 17 of 20 (85%) CCl4-PH rats and in 4 of 20 (20%) control rats. Bacteria were found in ascites in the experimental group (7/20, 35%) while no bacteria were found in the control group. All bacteria isolated from MLNs and ascites were Gram-negative. No bacteria were isolated from blood, spleen or liver.
Plasma levels of endotoxin (0.083±0.012 EU/mL) and ascites (0.062 ± 0.012 EU/mL) were significantly higher in the experimental group than in the control group (0.046 ± 0.009 EU/mL, P < 0.01).
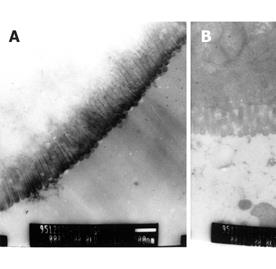
The distal ileum of rats with CCl4-induced portal hypertension did not differ grossly from that of the control rats. Clear and intact microvilli were seen in ileal enterocytes in the control rats. Shortened and thinned microvilli appeared in rats with CCl4-induced portal hypertension (Figure 1).
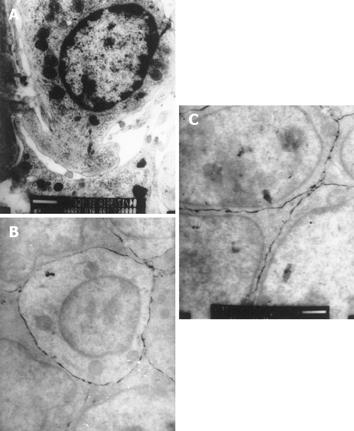
The tracer did not penetrate into the cells or intercellular juncture in the control rats. The tracer appeared between enterocytes and cellular junctions in rats with CCl4-induced portal hypertension. The penetrated tracer aggregated along the cellular junctions (tight or gap) or within the mucosal propria lamina without entering enterocytes or winding lines (Figure 2).
The mechanism underlying bacterial penetration into the intestinal barrier and entry into the systemic circulation in cirrhotic patients remains unclear[5-7]. Recent studies in animal model demonstrated that bacteria from the gastrointestinal tract can cross over the intestinal barrier to infect extraintestinal sites and/or the systemic circulation, a process known as bacterial translocation[8-10]. Various pathological changes such as damage of the intestinal barrier can increase the incidence of bacterial translocation[11]. Cirrhotic patients usually develop portal hypertension that causes intestinal congestion, thus inducing intestinal mucosal abnormalities[12], suggesting that portal hypertension may play a prominent role in the pathogenesis of spontaneous bacteremia and peritonitis in cirrhotic patients. Our results indicated that bacterial translocation occurred in 85% portal hypertensive rats, which was significantly higher (20%) than that in the control rats. Surgical manipulation and stress may be responsible for the translocation of bacteria[13-15].
The reason why permeability of the intestinal mucosa is increased remains unclear. In the present study, the tracer (Lanthanum) with a mean diameter of 4 nm is normally located outside the cell membrane, but may enter the cells through cellular pores, while their diameter exceeds 2 nm. Our results showed that the permeability of intestinal barrier was greatly increased in rats with portal hypertension. The tracer appeared between enterocytes and lamina propria cells, indicating that translocation occurs between enterocytes and lamina propria cells. This finding is consistent with the observation of Cole et al[17]. The damaged microvilli in rats with CCl4-induced portal hypertension demonstrated that permeability changes might occur before the development of pathological abnormality[15-17].
In conclusion, bacterial translocation occurs in rats with CCl4-induced portal hypertension and the increased permeability between epithelial cells contributes to the translocation.
S- Editor Wang XL L- Editor Elsevier HK E- Editor Kong LH
| 1. | Samonakis DN, Triantos CK, Thalheimer U, Patch DW, Burroughs AK. Management of portal hypertension. Postgrad Med J. 2004;80:634-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 505] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 3. | Garcia-Tsao G, Wiest R. Gut microflora in the pathogenesis of the complications of cirrhosis. Best Pract Res Clin Gastroenterol. 2004;18:353-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Hashimoto N, Ohyanagi H. Effect of acute portal hypertension on gut mucosa. Hepatogastroenterology. 2002;49:1567-1570. [PubMed] |
| 5. | Chiva M, Guarner C, Peralta C, Llovet T, Gómez G, Soriano G, Balanzó J. Intestinal mucosal oxidative damage and bacterial translocation in cirrhotic rats. Eur J Gastroenterol Hepatol. 2003;15:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Solà R, Soriano G. Why do bacteria reach ascitic fluid. Eur J Gastroenterol Hepatol. 2002;14:351-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Cirera I, Bauer TM, Navasa M, Vila J, Grande L, Taurá P, Fuster J, García-Valdecasas JC, Lacy A, Suárez MJ. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol. 2001;34:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 308] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 8. | Caly WR, Strauss E. A prospective study of bacterial infections in patients with cirrhosis. J Hepatol. 1993;18:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 334] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 9. | Sheer TA, Runyon BA. Spontaneous bacterial peritonitis. Dig Dis. 2005;23:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Ramachandran A, Prabhu R, Thomas S, Reddy JB, Pulimood A, Balasubramanian KA. Intestinal mucosal alterations in experimental cirrhosis in the rat: role of oxygen free radicals. Hepatology. 2002;35:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Deitch EA. Simple intestinal obstruction causes bacterial translocation in man. Arch Surg. 1989;124:699-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 173] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Groszmann RJ, Abraldes JG. Portal hypertension: from bedside to bench. J Clin Gastroenterol. 2005;39:S125-S130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Lora L, Mazzon E, Martines D, Fries W, Muraca M, Martin A, d'Odorico A, Naccarato R, Citi S. Hepatocyte tight-junctional permeability is increased in rat experimental colitis. Gastroenterology. 1997;113:1347-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Runyon BA, Borzio M, Young S, Squier SU, Guarner C, Runyon MA. Effect of selective bowel decontamination with norfloxacin on spontaneous bacterial peritonitis, translocation, and survival in an animal model of cirrhosis. Hepatology. 1995;21:1719-1724. [PubMed] |
| 15. | Alexander JW, Boyce ST, Babcock GF, Gianotti L, Peck MD, Dunn DL, Pyles T, Childress CP, Ash SK. The process of microbial translocation. Ann Surg. 1990;212:496-510; discussion 511-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 269] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Deitch EA, Bridges RM. Effect of stress and trauma on bacterial translocation from the gut. J Surg Res. 1987;42:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 69] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Cole GT, Seshan KR, Pope LM, Yancey RJ. Morphological aspects of gastrointestinal tract invasion by Candida albicans in the infant mouse. J Med Vet Mycol. 1988;26:173-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |